BiologicalBindingAssayReagent (Especially with active component for greatly improving immunological binding ability(binding/immobilizing cells(red blood cells, platelets, neutrophils, all relevant blood group antigens, such as Duffy and Kidd blood group systems, etc.) or proteins(antibodies,viral proteins,etc)) water soluble dark-greenish powder, pure 25g
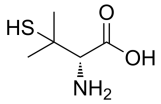
Penicillamine, D-Penicillamine, 3,3-Dimethyl-D-cysteine, 3-Mercapto-D-valine, D-(−)-2-Amino-3-mercapto-3-methylbutanoic acid, C5H11NO2S CAS NO.52-67-5 MW149.21 Beilstein:1722375 UNSPSC Code:41116107, Assay 98%(NMR), form solid color white to off-white, 1H NMR Spectrum consistent with structure, mp210 °C (dec.) (lit.), application(s) pharmaceutical (small molecule), format neat, storage temp 2-8DC, General description This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia. Application Penicillamine USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as: Penicillamine Capsules, Penicillamine Tablets. Analysis Note These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
2-Acetamido-3-sulfanylpropanoic acid, N-Acetyl-DL-cysteine, C5H9NO3S CAS No.7218-04-4 MW163.19486, form powder color White to off-white, 1H NMR Spectrum Consistent with structure, MS: Consistent with structure, Purity (NMR): ≥97.0%, storage 2-8°C, stored under nitrogen,

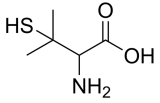
DL-Penicillamine, (±)-Penicillamine, 3-sulfanylvaline, C5H11NO2S CAS NO.52-66-4 MW149.22, Assay ≥98%(NMR) form solid powder, color Off-white to pale purple, 1H NMR Spectrum: Consistent with structure, Storage: Storage temp. 2-8°C
Fmoc-Cys(Mmt)-OH, Fmoc-S-4-methoxytrityl-L-cysteine; N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-S-[(4-methoxyphenyl)diphenylmethyl]-L-cysteine, N-α-Fmoc-S-p-methoxytrityl-L-cysteine, C38H33NO5S CAS NO.177582-21-7 MW615.74 UNSPSC Code:12352209, form solid, color White to off-white, 1H NMR Spectrum: Consistent with structure, Assay: ≥99%(HPLC), Optical Rotation: 16.033°(C=0.01g/ml DMF), application(s) peptide synthesis, functional group thiol, Storage: 2-8°C, stored under nitrogen, General description Building block for Fmoc SPPS which enables selective deprotection of cysteinyl thiol group on the solid phase. The Mmt group can be removed on the solid phase with 1% TFA in DCM containing 5% TIS [1,2,3,4]. Reference 4 describes a novel method for on-resin disulfide bond formation which uses Cys(Mmt) in combination with Cys(tBuS). Associated Protocols and Technical Articles Cleavage and Deprotection Protocols for Fmoc SPPS Fmoc SPPS of Cysteine-Containing Peptides. Literature references [1] K. Barlos, et al. in ′Peptides 1992, Proc. 22nd European Peptide Symposium′, C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 283. [2] C. Kellenberger, et al. (1995) Pept. Res., 8, 321. [3] K. Barlos, et al. (1996) Int. J. Peptide Protein Res., 47, 148. [4] A. K. Galande, et al. (2005) J. Comb. Chem., 7, 174. Application An Optimized Scalable Fully Automated Solid-Phase Microwave-Assisted cGMP-Ready Process for the Preparation of Eptifibatide: Details the use of Fmoc-Cys(Mmt)-OH in the synthesis of Eptifibatide, highlighting its role in a cGMP-ready process (Sabatino et al., 2020).
-OH.png)
Fmoc-Cys(Trt)-OH, N-(9-Fluorenylmethoxycarbonyl)-S-trityl-L-cysteine, Nα-Fmoc-S-trityl-L-cysteine, C37H31NO4S CAS NO.103213-32-7 MW585.71 Beilstein No.:4221286, form solid powder, color White to off-white, 1H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.03%, OR(C=1.02g/100mL, DMF): 21.5°, mp164-175 °C, application(s) peptide synthesis, functional group thiol, Storage: Storage temp. 2-8°C, General description High purity Fmoc-protected amino acid for research and process production of peptides, with very low levels of dipeptide, free-amino acids and acetic acid impurities. The standard derivative for Fmoc SPPS of peptides containing Cys [1]. The Trt group is removed with 95% TFA containing 1-5% TIS. Ideally, this derivative should be introduced using the symmetrical anhydride or DIPCDI/HOBt activation [2,3] to minimize enantiomerization. If activation with uronium or phosphonium reagents, such as HBTU or PyBOP®, is to be employed, it is strongly recommended that collidine is used as the base [4], as this has been shown to significantly reduce loss of optical integrity during coupling. Associated Protocols and Technical Articles Fmoc-amino acids for Peptide Production Cleavage and Deprotection Protocols for Fmoc SPPS Fmoc SPPS of Cysteine-Containing Peptides Literature references [1] S. N. McCurdy (1989) Pept. Res., 2, 147. [2] T. Kaiser, et al. (1996) Tetrahedron Lett., 37, 1187. [3] Y. X. Han, et al. (1997) J. Org. Chem., 62, 4307. [4] Y. N. Angell (2002) J. Peptide Res., 5, 292. Application On-resin synthesis of cyclic peptides via tandem N-to-S acyl migration and intramolecular thiol additive-free native chemical ligation: Discusses the use of Fmoc-Cys(Trt)-OH in the synthesis of cyclic peptides, highlighting the efficiency of the resin synthesis method. (Serra et al., 2020). Selective Bi‐directional Amide Bond Cleavage of N‐Methylcysteinyl Peptide: The study utilized Fmoc-Cys(Trt)-OH in exploring selective bi-directional amide bond cleavage in peptides, providing insights into controlled peptide modification. (Qiu et al., 2014).
-OH.png)
Boc-Cys(Me)-OH, C9H17NO4S CAS NO.16947-80-1 MW235.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=1.01 g/100ml acetic): -27.8°, Storage: 2-8°C, stored under nitrogen,
-OH.png)
(2s)-2-Amino-2-methyl-3-sulfanylpropanoic acid hydrochloride, α-Me-D-Cys-OH·HCl, 2-amino-3-mercapto-2-methylpropanoic acid hydrochloride, C4H10ClNO2S, CAS NO.151062-55-4 MW171.65, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Storage: 2-8°C, stored under nitrogen,
-2-Amino-2-methyl-3-sulfanylpropanoic acid hydrochloride.png)
N-(((9H-Fluoren-9-yl)methoxy)carbonyl)-S-(tert-butylthio)-L-cysteine, Nα-Fmoc-S-tert-butylthio-L-cysteine, Fmoc-Cys(StBu)-OH, Fmoc-Cys(tButhio)-OH, C22H25NO4S2 CAS NO.73724-43-3 MW431.57, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 98.15%, mp73-77 °C, Storage: Storage temp. 2-8°C, application(s) peptide synthesis
methoxy)carbonyl)-S-(tert-butylthio)-L-cysteine.png)
N,N′-Dibenzoyl-L-cystine, C20H20N2O6S2 CAS No.25129-20-8 MW448.51, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.79%, Storage: 2-8°C, stored under nitrogen

(2R,2'R)-Di-tert-butyl 3,3'-disulfanediylbis(2-aminopropanoate) dihydrochloride, C14H30Cl2N2O4S2 CAS No. 38261-78-8 MW425.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR( C=0.97 g/100mL,MEOH ): -105.00°, Storage: Storage temp. 2-8°C
-Di-tert-butyl 3,3'-disulfanediylbis(2-aminopropanoate) dihydrochloride.png)
Fmoc-D-Cys(Trt)-OH, C37H31NO4S CAS NO.167015-11-4 MW585.71, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Enantiomeric Excess: 99.37%, Assay (HPLC): 98.64%, Water(KF): 0.26%, OR(C=1.00 g/100ml CHCL3): -15.6°, Storage: Storage temp. 2-8°C
-OH.png)
L-Cysteine methyl ester (hydrochloride), C4H10ClNO2S CAS No.18598-63-5 MW171.65, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation[a]: 0.3860°(C=1.0038g/ml MEOH), Storage: 2-8°C, sealed storage, away from moisture
.png)
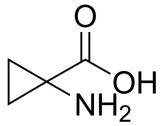
1-Aminocyclopropane-1-carboxylic acid, C4H7NO2 CAS NO.22059-21-8 MW101.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.68%, Storage: Store at room temperature
(R)-2-Aminopent-4-ynoic acid, C5H7NO2 CAS NO.23235-03-2 MW113.11, form Solid, color Light brown to brown, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature
-2-Aminopent-4-ynoic acid.png)
(S)-2-Aminopent-4-ynoic acid, C5H7NO2 CAS No.23235-01-0 MW113.01, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
-2-Aminopent-4-ynoic acid.png)
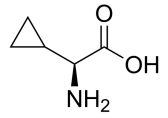
H-Cyclopropyl-Gly-OH, C5H9NO2 CAS NO.49606-99-7 MW115.13, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=1.0 g/100ml 1N/HCL): 100.5°, Storage: Storage temp. 2-8°C
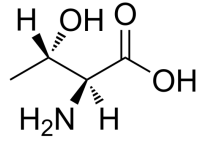
L-Allothreonine, H-allo-Thr-OH, CAS NO.28954-12-3 C4H9NO3 MW119.12, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
(S)-2-Amino-3-methoxypropanoic acid, C4H9NO3 CAS NO.32620-11-4 MW119.12, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR[α](C=1.00 g/100ml 1N/HCL): 17.2°, Storage: Store at room temperature
-2-Amino-3-methoxypropanoic acid.png)
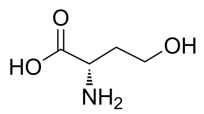
L-Homoserine, C4H9NO3 CAS NO.672-15-1 MW119.12, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.37%, Storage: Store at room temperature
(S)-3-Amino-4-hydroxybutanoic acid, C4H9NO3 CAS NO.16504-57-7 MW119.12, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
-3-Amino-4-hydroxybutanoic acid.png)
β-Cyclopropyl-D-Ala-OH, H-.beta.-Cyclopropyl-D-Ala-OH, C6H11NO2 CAS NO.121786-39-8 MW129.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structurePurity (NMR): ≥97.0%, Storage: Store at room temperature

Glycine tert-butyl ester, tert-Butyl glycinate, H2NCH2COOC(CH3)3 CAS NO.6456-74-2 MW131.17, Assay ≥92.5% (GC), form liquid, bp29-31 °C/3 hPa, density 0.96 g/cm3 at 20 °C, Identity (IR): passes test, storage temp.2-8°C

H-Thr(Me)-OH, C5H11NO3 CAS NO.4144-02-9 MW133.15, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation: -33.9°(C=0.01g/ml H2O), Storage: Storage temp. 2-8°C
-OH.png)
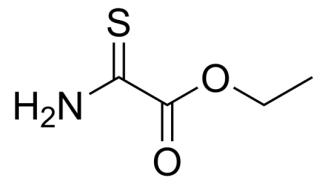
2-Thiooxamic acid ethyl ester, C4H7NO2S CAS NO.16982-21-1 MW133.17, form Solid, color Off-white to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (GC): 99.46%, Storage: Storage temp. 2-8°C
Methyl (R)-5-oxopyrrolidine-2-carboxylate, Methyl D-pyroglutamate, C6H9NO3 CAS NO.64700-65-8 MW143.14, form Viscous liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (GC): 99.61%, OR(C=0.66 g/100ml ETOH): -9.7°, Storage: Store at room temperature
-5-oxopyrrolidine-2-carboxylate.png)
(S)-2-Aminoheptanoic acid, S-2-Aminoheptanoic acid, C7H15NO2 CAS NO.44902-02-5 MW145.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Store at room temperature
-2-Aminoheptanoic acid.png)
3-Amino-4-methoxy-4-oxobutanoic acid, C5H9NO4 CAS NO.65414-77-9 MW147.13, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation: 3.5°(C=0.156 g/100ml, EA), Storage: Store at room temperature
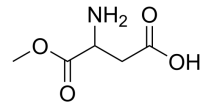
Methyl 1-aminocyclopropanecarboxylate hydrochloride, C5H10ClNO2 CAS NO.72784-42-0 MWW151.59, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
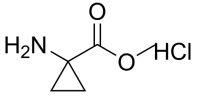
1-Aminocyclobutane-1-carboxylic acid hydrochloride, Cyclobutanecarboxylic acid, 1-amino-, hydrochloride; 1-Aminocyclobutane-1-carboxylic acid hydrochloride, C5H10ClNO2 CAS NO.98071-16-0 MW151.59, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
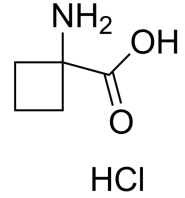
(S)-Methyl 2-(methylamino)propanoate hydrochloride, N-Methyl-L-alanine methyl ester HCl, C5H12ClNO2 CAS NO.20045-77-6 MW153.61, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%OR(C=1.13g/100ml ETOH): 1.4°, Storage: Store at room temperature
-Methyl 2-(methylamino)propanoate hydrochloride.png)
Methyl 2-aminoisobutyrate (hydrochloride), C5H12ClNO2 CAS NO.15028-41-8 MW153.61, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: -20°C, sealed storage, away from moisture
.png)
N-Formyl-L-leucine, N-Formyl-S-leucine; N-Formylleucine; NSC 334321, C7H13NO3 CAS NO.6113-61-7 MW159.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.70%, Storage: Storage temp. -20°C
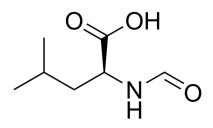
(R)-2-Aminohexanedioic acid, D-α-Aminohexanoic Diacid, C6H11NO4 CAS NO.7620-28-2 MW161.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Store at room temperature
-2-Aminohexanedioic acid.png)
H-D-Glu(OMe)-OH, C6H11NO4 CAS NO.6461-04-7 MW161.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.25%
-OH.png)
O-(tert-Butyl)-L-serine, C7H15NO3 CAS NO.18822-58-7 MW161.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
-L-serine.png)
H-D-SER(TBU)-OH, C7H15NO3 CAS NO.18783-53-4 MW161.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
-OH.png)
S-Allyl-D-cysteine, C6H11NO2S CAS NO.770742-93-3 MW161.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: 2-8°C, stored under nitrogen

2-(Benzylamino)acetic acid, C9H11NO2 CAS NO.17136-36-6 MW165.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Purity (NMR): ≥98.0%, Water(KF): 0.15%, Storage: Storage temp. 2-8°C
acetic acid.png)
Methyl 2-amino-2-cyclopropylacetate hydrochloride,C6H12ClNO2 CAS NO.535936-86-8 MW165.62, form Solid, color White to light brown, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: 2-8°C, sealed storage, away from moisture

Ethyl glutamate,C7H13NO4 CAS NO.1119-33-1 MW175.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature

H-D-Thr(tBu)-OH, O-(tert-butyl)-D-Threonine, C8H17NO3 CAS NO.201274-81-9 MW175.23, form powder, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation[a]: 41.9°(C=0.010024g/ml MEOH), Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
L-Homophenylalanine, C10H13NO2 CAS NO.943-73-7 MW179.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structurem Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Store at room temperature
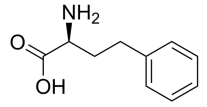
(R)-2-Amino-3-(m-tolyl)propanoic acid, C10H13NO2 CAS NO.114926-39-5 MW179.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.96%, OR(C=1.0g/100ml H2O): 31.0°, Storage: Storage temp. 2-8°C
-2-Amino-3-(m-tolyl)propanoic acid.png)
H-DL-Phe(4-Me)-OH, DL-4-methylphenylalanine, 4-methylphenylalanine, C10H13NO2 CAS NO.4599-47-7 MW179.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=1 g/100ml 0.5N HCL): -0.54°, mp277 °C, bp323.5°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-859.
-OH.png)
Ethyl 1-aminocyclobutanecarboxylate hydrochloride, C7H14ClNO2 CAS NO.145143-60-8 MW179.64, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C

Methyl 1-aminocyclopen tanecarboxylate hydrochloride, C7H14ClNO2 CAS NO.60421-23-0 MW179.64, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature

H-cis-Hyp-OMe (hydrochloride), methyl 4-hydroxy-2-pyrrolidinecarboxylate hydrochloride, C6H12ClNO3 CAS NO.40126-30-5 MW181.62, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp100.9-101 °C, Storage: Storage temp. 2-8°C, [1]. Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337. [2]. Nalawansha DA, et al. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem Biol. 2020;27(8):998-985.
.png)
(4S)-4-Hydroxy-D-proline methyl ester (hydrochloride), C6H12ClNO3 CAS NO.481704-21-6 MW181.62, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.07%, Optical Rotation: 25.6493° (C=0.4698 g/100ml, MEOH, 20°C, 589nm), Storage: 2-8°C, sealed storage, away from moisture
-4-Hydroxy-D-proline methyl ester (hydrochloride).png)
4-Aminotetrahydro-2H-pyran-4-carboxylic acid hydrochloride, 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL, C6H12ClNO3 CAS NO.217299-03-1, MW181.62, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature

H-Phe(3-F)-OH, 3-Fluoro-L-phenylalanine; L-3-Fluorophenylalanine; m-Fluoro-L-phenylalanine; H-m-Fluoro-Phe-OH; H-Phe(3-F)-OH, C9H10FNO2 CAS NO.19883-77-3 MW183.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Store at room temperature
-OH.png)
H-D-Phg(4-Cl)-OH, (R)-4-Chlorophenylglycine, C8H8ClNO2 CAS NO.43189-37-3 MW185.61, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.82%, mp272-274 °C, bp328.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
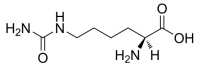
L-Homocitrulline, C7H15N3O3 CAS NO.1190-49-4 MW189.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
N-tert-Butoxycarbonyl-D-alanine, C8H15NO4 CAS NO.7764-95-6 MW189.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=1.00g/100ml MEOH): 25.0°, Water(KF): 0.68%, Residue on Ignition: 0.03%, Storage: Storage temp. 2-8°C

(R)-2-Amino-3-(3-cyanophenyl)propanoic acid, CAS NO.263396-43-6 MW190.202, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.26%, Optical Rotation: -8.7° (C=0.01 g/ml, 1N NAOH), Storage: Store at room temperature
-2-Amino-3-(3-cyanophenyl)propanoic acid.png)
(S)-L-DABA (dihydrochloride), (S)-L-2,4-Diaminobutyric acid (dihydrochloride), CAS NO.1883-09-6 MW191.06, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, OR(C=1.00 g/100ml H2O): 6.2°, Storage: 2-8°C, sealed storage, away from moisture
-L-DABA (dihydrochloride).png)
(S)-Methyl 2-amino-2-cyclopentylacetate hydrochloride, C8H16ClNO2 CAS NO.14328-62-2 MW193.67, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml MEOH): 22.9°, Storage: Storage temp. 2-8°C
-Methyl 2-amino-2-cyclopentylacetate hydrochloride.png)
O-Benzyl-DL-serine, C10H13NO3 CAS NO.5445-44-3 MW195.218, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.31%, Storage: 2-8°C, protect from light

α-Methyl-p-tyrosine, C10H13NO3 CAS NO.658-48-0 MW195.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 96.97%, Storage: Storage temp. 2-8°C

3-Amino-3-(4-methoxyphenyl)propanoic acid, C10H13NO3 CAS NO.5678-45-5 MW195.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.90%, Storage: RT, protect from light
propanoic acid.png)
H-Ser(Bzl)-OH, O-Benzyl-L-serine, C10H13NO3 CAS NO.4726-96-9 MW195.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.95%, Storage: Storage temp. 2-8°C
-OH.png)
Ethyl (2S,4R)-4-hydroxypyrrolidine-2-carboxylate hydrochloride, Ethyl trans-4-hydroxy-L-prolinate hydrochloride, C7H14ClNO3 CAS NO.33996-30-4 MW195.64, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature
-4-hydroxypyrrolidine-2-carboxylate hydrochloride.png)
(2S,4S)-4-Cyclohexylpyrrolidine-2-carboxylic acid, C11H19NO2 CAS NO.103201-78-1 MW197.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation[a]: -30.2°(C=0.011192 g/mL ACOH), Storage: Store at room temperature
-4-Cyclohexylpyrrolidine-2-carboxylic acid.png)
4-Chloro-L-phenylalanine, C9H10ClNO2 CAS NO.14173-39-8 MW199.63, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C

N-(tert-Butoxycarbonyl)-N-methyl-D-alanine, C9H17NO4 CAS NO.19914-38-6 MW203.238, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation: 26.1°(C=0.01g/mL,ETOH, 20°C, 589nm), Storage: Storage temp. 2-8°C
-N-methyl-D-alanine.png)
N-tert-Butoxycarbonyl-N-methylalanine,C9H17NO4 CAS NO.16948-16-6 MW203.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.24%, Storage: Storage temp. 2-8°C

2-((tert-Butoxycarbonyl)amino)-2-methylpropanoic acid, C9H17NO4 CAS NO.30992-29-1 MW203.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
amino)-2-methylpropanoic acid.png)
(R)-Methyl 2-((tert-butoxycarbonyl)amino)propanoate, C9H17NO4 CAS NO.91103-47-8 MW203.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.22%, Optical Rotation: 42.03° (C=0.01 g/ml, MEOH), Storage: Store at room temperature
-Methyl 2-((tert-butoxycarbonyl)amino)propanoate.png)
Ethyl (tert-Butoxycarbonyl)glycinate, C9H17NO4 CAS NO.14719-37-0 MW203.24, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (GC): 99.11%, Water(KF): 0.24%, Storage: Storage temp. 2-8°C
glycinate.png)
H-D-Glu(OtBu)-OH, D-GLUTAMIC ACID-Y-T-BUTYLESTER, C9H17NO4 CAS NO.45125-00-6 MW203.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature
-OH.png)
DL-Boc-Aminobutyric acid, Boc-2-aminobutyric acid, C9H17NO4 CAS NO.77284-64-1 MW203.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature

Boc-D-2,3-diaminopropionic acid, N-t-Boc-amino-D-alanine, Boc-D-Dap-OH, C8H16N2O4 CAS NO.76387-70-7 MW204.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature

Sodium 2-acetamidomalonate, Propanedioic acid, (acetylamino)-, disodium salt; Acetamidomalonic acid (disodium salt), C5H5NNa2O5 CAS NO.117976-12-2 MW205.08, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: 2-8°C, sealed storage, away from moisture,

H-D-Phg-OtBu, H-D-PHG-OTBU HCL, (R)-2-Amino-2-phenylacetic acid tert-butyl ester, C12H17NO2 CAS NO.65715-93-7 MW207.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): ≥98%, Storage: Storage temp. -20°C

(S)-2-Amino-3-(4-nitrophenyl)propanoic acid,H-p-Nitro-Phe-OH, C9H10N2O4 CAS NO.949-99-5 MW210.19, form Solid, color Off-white to light brown, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Store at room temperature
-2-Amino-3-(4-nitrophenyl)propanoic acid.png)
(R)-Methyl 2-amino-3-(tert-butoxy)propanoate hydrochloride, C8H18ClNO3 CAS NO.78537-14-1 MW211.69, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
-Methyl 2-amino-3-(tert-butoxy)propanoate hydrochloride.png)
(R)-2-((tert-Butoxycarbonyl)amino)pent-4-ynoic acid, C10H15NO4 CAS NO.63039-46-3 MW213.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥95.0%, Optical Rotation[a]: -32.6364°(C=1.1311 g/100ml CHCL3), Storage: Storage temp. 2-8°C
-2-((tert-Butoxycarbonyl)amino)pent-4-ynoic acid.png)
H-2-Nal-OH, 3-(2-Naphthyl)-L-alanine, C13H13NO2 CAS NO.58438-03-2 MW215.252, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Enantiomeric Excess: 100.00%, Assay (NMR): ≥98.0%, Storage: Storage temp. 2-8°C

H-Dab(Boc)-OH, (S)-2-Amino-4-((tert-butoxycarbonyl)amino)butanoic acid, C9H18N2O4 CAS NO.10270-94-7 MW218.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature
-OH.png)
2-Amino-2-(3-(trifluoromethyl)phenyl)acetic acid, C9H8F3NO2 CAS NO.242475-26-9 MW219.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.41%, Storage: Storage temp. 2-8°C
phenyl)acetic acid.png)
Boc-L-Homoserine, N-(tert-Butoxycarbonyl)-L-homoserine, C9H17NO5 CAS NO.41088-86-2 MW219.23, form Solid, color White to gray, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Storage temp. 2-8°C

(R)-Methyl 2-(tert-butoxycarbonylamino)-3-hydroxypropanoate, N-(tert-Butoxycarbonyl)-D-serine Methyl Ester, C9H17NO5 CAS NO.95715-85-8 MW219.237, form Liquid, color Colorless to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.45%, Optical Rotation[a]: 31.7775°(C=1g/100ml solvent:MEOH), Storage: Storage temp. 2-8°C
-Methyl 2-(tert-butoxycarbonylamino)-3-hydroxypropanoate.png)
N-Benzyl-L-isoleucine, C13H19NO2 CAS NO.1859-49-0 MW221.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 97.29%, Storage: Storage temp. 2-8°C

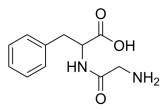
Glycyl-DL-phenylalanine, C11H14N2O3 CAS NO.721-66-4 MW222.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C
Z-Sar-OH, N-Carbobenzoxy-N-methylglycine, C11H13NO4 CAS NO.39608-31-6 MW223.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.06%, Residue on Ignition: 0.06%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

tert-Butyl D-leucinate hydrochloride, C10H22ClNO2 CAS NO.13081-32-8 MW223.74, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.32%, OR[α](C=1.21 g/100ml EtOH): -18.0°, Storage: Storage temp. 2-8°C

Ethyl 2-(benzylamino)acetate hydrochloride, C11H16ClNO2 CAS NO.6344-42-9 MW229.70, form Solid, color White to off-white, Assay (HPLC): 95.45%, Storage: Storage temp. 2-8°C
acetate hydrochloride.png)
(2S)-2-[[(tert-Butoxy)carbonyl](methyl)amino]-3-methylbutanoic acid, N-(tert-Butoxycarbonyl)-N-methyl-L-valine, N-Boc-N-methyl-L-valine, C11H21NO4 CAS NO.45170-31-8 MW231.292, form <45°C Solid,>60°C Liquid, color Colorless to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.21%, Storage: Storage temp. 2-8°C
-2-[[(tert-Butoxy)carbonyl](methyl)amino]-3-methylbutanoic acid.png)
Boc-Ala-NMe(OMe), (S)-2-((tert-Butoxycarbonyl)amino)-N-methoxy-N-methylpropanamide, (S)-2-(tert-Butoxycarbonylamino)-N-methoxy-N-methylpropionamide, N-(tert-Butoxycarbonyl)-L-alanine N'-Methoxy-N'-methylamide, C10H20N2O4 CAS NO.87694-49-3 MW232.28, form Solid, color White to off-white, Assay (HPLC): 98.63%, mp:148-153°C, Storage: 2-8°C, sealed storage, away from moisture and light, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
.png)
Ethyl (S)-2,5-diaminopentanoate dihydrochloride, L-Ornithineethylesterhydrochloride, C7H18Cl2N2O2 CAS NO.84772-29-2 MW233.14, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature
-2,5-diaminopentanoate dihydrochloride.png)
4-(Trifluoromethyl)-L-phenylalanine, C10H10F3NO2 CAS NO.114926-38-4 MW233.19, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.65%, Optical Rotation[a]: -16.23°(C=0.01g/ml MEOH), Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-807.
-L-phenylalanine.png)
N-Boc-trans-4-fluoro-L-proline, (2S,4R)-1-(tert-Butoxycarbonyl)-4-fluoro-2-pyrrolidinecarboxylic Acid, C10H16FNO4 CAS NO.203866-14-2 MW233.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp115-119°C, bp346°C at 760 mmHg, Storage: Storage temp. 2-8°C

Boc-Thr(Me)-OH, 2-[(tert-butoxycarbonyl)amino]-3-methoxybutanoic acid, C10H19NO5 CAS NO.48068-25-3 MW233.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.14%, OR(C=1.42g/100mL, MEOH): 9.86°, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
Methyl 2-amino-3-(4-fluorophenyl)propanoate hydrochloride, P-FLUORO-DL-PHENYLALANINE-OME HCL, C10H13ClFNO2 CAS NO.64282-12-8 MW233.67, mp176.5-178.5 °C, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.52%, Storage: Store at room temperature
propanoate hydrochloride.png)
1-(Benzyloxycarbonylamino)cyclopropyl-1-carboxylic acid, 1-(Carbobenzoxyamino)cyclopropanecarboxylic acid, N-Cbz-1-aminocyclopropanecarboxylic acid, C12H13NO4 CAS NO.84677-06-5 MW235.24, mp157.0-161.0 °C, bp449.0±34.0 °C at 760 mmHg, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.50%, Storage: Store at room temperature
cyclopropyl-1-carboxylic acid.png)
(2S,3S)-2-(Benzyl(methyl)amino)-3-methylpentanoic acid, C14H21NO2 CAS NO.4125-97-7 MW235.32, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 97.84%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

Z-N-Me-Ala-OH, N-[(benzyloxy)carbonyl]-N-methylalanine, C12H15NO4 CAS NO.21691-41-8 MW237.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.63%, mp54-56°C, bp423 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1136. [2]. Bioorganic and Medicinal Chemistry Letters, vol. 10, # 12 p. 1361-1363. [3]. Tetrahedron, vol. 2, p. 211,226 Acta Chimica Academiae Scientiarum Hungaricae,, vol. 6, p.219,226

H-D-Glu(OBzl)-OH, 5-Benzyl D-Glutamate, C12H15NO4 CAS NO.2578-33-8 MW237.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.93%, mp158 °C, bp426.1 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
(R)-Phenylglycine tert-butyl ester hydrochloride, C12H18ClNO2 CAS NO.256478-95-2 MW243.73, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

(S)-2-Amino-3-(2-bromophenyl)propanoic acid, ortho-bromo-L-phenylalanine; 2-Bromo-L-phenylalanine, C9H10BrNO2 CAS NO.42538-40-9 MW244.09, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp270°C, bp363.2°C at 760 mmHg, Storage: Store at room temperature
-2-Amino-3-(2-bromophenyl)propanoic acid.png)
(S)-2-Amino-3-(4-bromophenyl)propanoic acid, 4-Bromo-L-phenylalanine, (S)-p-Bromophenylalanine; L-4-Bromophenylalanine; L-p-Bromophenylalanine; p-Bromo-L-phenylalanine, C9H10BrNO2 CAS NO.24250-84-8 MW244.09, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.91%, mp265°C, bp368.4°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-807. [2]. B J Allen, et al. Dose fractionation in neutron capture therapy for malignant melanoma. Basic Life Sci. 1989:50:63-7. [3]. B J Allen, et al. Thermal neutron capture therapy: the Japanese-Australian clinical trial for malignant melanoma. Basic Life Sci. 1989:50:69-73. [4]. Inchan Kwon, et al. Design of a bacterial host for site-specific incorporation of p-bromophenylalanine into recombinant proteins. J Am Chem Soc. 2006 Sep 13;128(36):11778-83. [5]. J A Coderre, et al. Neutron capture therapy for melanoma. Basic Life Sci. 1989:50:219-32.
-2-Amino-3-(4-bromophenyl)propanoic acid.png)
2-Amino-3-(4-bromophenyl)propanoic acid, 4-Bromo-DL-phenylalanine, C9H10BrNO2 CAS NO.14091-15-7 MW244.088, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.95%, Optical Rotation: 0.60°(C=0.01g/mL, HCL, 20°C, 589nm), mp262-263°C, bp368.4±32.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-896. [2]. B J Allen, et al. Dose fractionation in neutron capture therapy for malignant melanoma. Basic Life Sci. 1989:50:63-7. [3]. B J Allen, et al. Thermal neutron capture therapy: the Japanese-Australian clinical trial for malignant melanoma. Basic Life Sci. 1989:50:69-73. [4]. Inchan Kwon, et al. Design of a bacterial host for site-specific incorporation of p-bromophenylalanine into recombinant proteins. J Am Chem Soc. 2006 Sep 13;128(36):11778-83. [5]. J A Coderre, et al. Neutron capture therapy for melanoma. Basic Life Sci. 1989:50:219-32.
propanoic acid.png)
4-((tert-Butoxycarbonyl)amino)piperidine-4-carboxylic acid, N-BOC-AMINO-PIPERIDINYL-1,1-CARBOXYLIC ACID, C11H20N2O4 CAS NO.252720-31-3 MW244.29, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, bp410 °C at 760 mmHg, Storage: 2-8°C, protect from light
amino)piperidine-4-carboxylic acid.png)
Leu-Leu-OH, L-Leucyl-L-leucine, (S)-2-((S)-2-Amino-4-methylpentanamido)-4-methylpentanoic Acid, C12H24N2O3 CAS NO.3303-31-9 MW244.33, mp270-272 °C, bp432.7°C at 760 mmHg, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation[a]: -12.0°(C=0.01 g/ml 1N NaOH), Storage: -80°C, 2 years; -20°C, 1 year (Sealed storage, away from moisture), [1]. A van Boven, et al. Utilization of dipeptides by Lactococcus lactis ssp. cremoris. Biochimie. 1988 Apr;70(4):535-42. [2]. Balraj Doray, et al. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007 May;18(5):1887-96. [3]. Kazuteru Fukasawa, et al. A novel compound, NK150460, exhibits selective antitumor activity against breast cancer cell lines through activation of aryl hydrocarbon receptor. Mol Cancer Ther. 2015 Feb;14(2):343-54. [4]. Lesley Colgan, et al. Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter. Traffic. 2007 May;8(5):512-22. [5]. M Plauth, et al. Nitrogen absorption from isonitrogenous solutions of L-leucyl-L-leucine and L-leucine: a study in the isolated perfused rat small intestine. Clin Sci (Lond). 1992 Mar;82(3):283-90.

Methyl (tert-butoxycarbonyl)-L-leucinate, C12H23NO4 CAS NO.63096-02-6 MW245.32, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp147-149 °C, bp322.5°C at 760 mmHg, Flash point 113°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-L-leucinate.png)
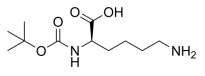
Boc-D-Lys-OH, Nα-(tert-Butoxycarbonyl)-D-lysine, C11H22N2O4 CAS NO.106719-44-2 MW246.30, form Solid, color White to off-white, Assay: ≥98.0%, mp198°C, bp410.5°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
Boc-Asp(OMe)-OH, Boc-L-aspartic Acid beta-Methyl Ester, C10H17NO6 CAS NO.59768-74-0 MW247.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp71 °C, bp411°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
2-((tert-Butoxycarbonyl)amino)-3-ethoxy-3-oxopropanoic acid, 2-(N-BOC-AMINO)MALONIC ACID MONOETHYL ESTER, C10H17NO6 CAS NO.137401-45-7 MW247.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp98-99°C, bp397.2±37.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
amino)-3-ethoxy-3-oxopropanoic acid.png)
BOC-D-GLU-OH, N-(tert-Butoxycarbonyl)-D-glutamic Acid, C10H17NO6 CAS NO.34404-28-9 MW247.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Optical Rotation[a]: 15.5°(C=0.009914g/ml MEOH), mp108 °C, bp435.9±35.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
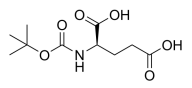
Z-DL-Pro-OH,N-Cbz-DL-proline, DL-Cbz-Proline,C13H15NO4 CAS NO.5618-96-2 MW249.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 96.88%, mp101-102 °C, bp432.3°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Matthew BAGGOTT, et al. Advantageous tryptamine compositions for mental disorders or enhancement. WO2022061242A1
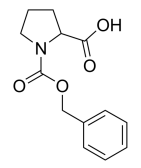
(tert-Butoxycarbonyl)-L-methionine, N-(tert-Butoxycarbonyl)-L-methionine, C10H19NO4S CAS NO.2488-15-5 MW249.33, form <47°C Solid,>53°C Liquid, color Colorless to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation: -17.0° (C=1.00 g/100ml, MEOH), Water(KF): 0.80%, mp47-53 °C, bp415.5 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. [2]. Griet Van Zeebroeck, et al. Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. Nat Chem Biol. 2009 Jan;5(1):45-52.
-L-methionine.png)
Boc-D-Met-OH, N-(tert-Butoxycarbonyl)-D-methionine, C10H19NO4S CAS NO.5241-66-7 MW249.33, form <47°C Solid,>50°C Liquid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp47-50°C, bp415.5°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

1-(tert-Butyl) 5-methyl L-glutamate hydrochloride, C10H20ClNO4 CAS NO.34582-33-7 MW253.72, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00 g/100ml, Ethanol): 17.8°, Storage: 2-8°C, sealed storage, away from moisture, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
 5-methyl L-glutamate hydrochloride.png)
H-Glu(OtBu)-OMe.HCl, 5-tert-Butyl 1-Methyl L-Glutamate Hydrochloride, L-Glutamic acid 5-tert-butyl 1-methyl ester hydrochloride, C10H20ClNO4 CAS NO.6234-01-1 MW253.72, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation[a]: 26.7°(C=0.01g/ml MEOH), Storage: 2-8°C, sealed storage, away from moisture, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OMe.HCl.png)
Boc-Hyp-OEt, 1-(tert-butyl) 2-ethyl 4-hydroxypyrrolidine-1,2-dicarboxylate, C12H21NO5 CAS NO.37813-30-2 MW259.30, form Viscous Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

3-Bromo-L-tyrosine, 3-Bromo-Tyr, C9H10BrNO3 CAS NO.38739-13-8 MW260.08, form Solid, color Off-white to light brown, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation[a]: -18.568°(C=0.01g/ml H2O), mp247-248 °C, bp402.8±45.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

Boc-Ala-Ala-OH, (tert-Butoxycarbonyl)-L-alanyl-L-alanine, N-Boc-L-alanyl-L-alanine, C11H20N2O5 CAS NO.27317-69-7 MW260.29, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml MEOH): -38.0°, mp132-133 °C, bp483.5±30.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Norris Timothy, et al. Preparation of polymorphs of the prodrug 6-N-(L-Ala-L-Ala)-trovafloxacin methanesulfonate. Patent WO9708191.

H-Arg-OMe.2HCL, L-Arginine methyl ester dihydrochloride, methyl 2-amino-5-guanidinopentanoate dihydrochloride, C7H18Cl2N4O2 CAS NO.26340-89-6 MW261.15, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.03%, Residue on Ignition: 0.12%, OR[α](C=1.556 HO2): 17.2°, mp190°C, bp329.9°C at 760 mmHg, flash point: 153.3°C, Storage: Storage temp. 2-8°C, [1]. Minoru Yamaguchi, et al. Enhancement of MALDI-MS spectra of C-terminal peptides by the modification of proteins via an active ester generated in situ from an oxazolone. Anal Chem. 2006 Nov 15;78(22):7861-9. [2]. Mohammed A Nayeem, et al. Modulation by salt intake of the vascular response mediated through adenosine A(2A) receptor: role of CYP epoxygenase and soluble epoxide hydrolase. Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R325-33. [3]. Peter G W Gettins, et al. A proximal pair of positive charges provides the dominant ligand-binding contribution to complement-like domains from the LRP (low-density lipoprotein receptor-related protein). Biochem J. 2012 Apr 1;443(1):65-73. [4]. Shizuka Hartenbach, et al. An engineered L-arginine sensor of Chlamydia pneumoniae enables arginine-adjustable transcription control in mammalian cells and mice. Nucleic Acids Res. 2007;35(20):e136. [5]. Suzan D Santos, et al. Shrimp waste extract and astaxanthin: rat alveolar macrophage, oxidative stress and inflammation. J Food Sci. 2012 Jul;77(7):H141-6.

Boc-D-Ser(tBu)-OH, C12H23NO5 CAS NO.248921-66-6 MW261.32, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥95.0%, bp383.1±37.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
N-((Benzyloxy)carbonyl)-N-methyl-L-valine,(S)-N-(Benzyloxycarbonyl)-N-methylvaline, C14H19NO4 CAS NO.42417-65-2 MW265.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.46%, OR(C=1.03g/100ml ETHANOL): -86.4°, mp68-70°C, bp407.1 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
carbonyl)-N-methyl-L-valine.png)
2-Amino-3-(2-oxo-1,2-dihydroquinolin-4-yl)propanoic acid hydrochloride, 3-(2-Oxo-1,2-dihydro-4-quinolinyl)-DL-alanine Hydrochloride, N-des(4-Chlorobenzoyl) Rebamipide, C12H13ClN2O3 CAS NO.4876-14-6 MW268.70, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, mp220-221 °C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1117.
propanoic acid hydrochloride.png)
(S)-2-((tert-Butoxycarbonyl)amino)-3-cyclohexylpropanoic acid, Boc-Cha-OH, C14H25NO4 CAS NO.37736-82-6 MW271.35, form <40°C Solid,>42°C Liquid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.07g/100ml MEOH): -9.5°, mp40-42°C, bp420.4°C at 760 mmHg, Flash point 208.1°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1068.
-2-((tert-Butoxycarbonyl)amino)-3-cyclohexylpropanoic acid.png)
(R)-2-tert-Butoxycarbonylamino-pentanedioic acid dimethyl ester, D-Glutamic acid, N-[(1,1-dimethylethoxy)carbonyl]-, dimethyl ester, dimethyl(tert-butoxycarbonyl)-D-glutamate, C12H21NO6 CAS NO.130622-05-8 MW275.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.02%, bp370.9±32.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-2-tert-Butoxycarbonylamino-pentanedioic acid dimethyl ester.png)
Boc-Glu(OMe)-OMe, Dimethyl N-(tert-Butoxycarbonyl)-L-glutamate, N-Boc-L-glutamic Acid 1,5-Dimethyl Ester, C12H21NO6 CAS NO.59279-60-6 MW275.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp43.0-47.0 °C, bp370.9°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OMe.png)
N-Boc-L-phenylalanine methyl ester, N-(tert-Butoxycarbonyl)-L-phenylalanine Methyl Ester, C15H21NO4 CAS NO.51987-73-6 MW279.33, form Solid, form White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp36-40°C, bp403.7°C at 760 mmHg, Flash point 113°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-897.

Methyl (tert-butoxycarbonyl)-D-phenylalaninate, C15H21NO4 CAS NO.77119-84-7 MW279.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 96.68%, mp51 °C, bp403.7±38.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-961.
-D-phenylalaninate.png)
H-Lys(Z)-OH, Nε-Carbobenzoxy-L-lysine, N6-(Benzyloxycarbonyl)-L-lysine, C14H20N2O4 CAS NO.1155-64-2 MW280.324, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Enantiomeric Excess: 100%, Assay (HPLC): 99.84%, Water(KF): 0.36%, mp259°C, bp499.6°C at 760 mmHg, Residue on Ignition: 0.39%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. [2]. J Zheng, et al. Production of microspheres with surface amino groups from blends of Poly(Lactide-co-glycolide) and Poly(epsilon-CBZ-L-lysine) and use for encapsulation. Biotechnol Prog. 1999 Jul-Aug;15(4):763-7. [3]. J Zheng, et al. Transfection of cells mediated by biodegradable polymer materials with surface-bound polyethyleneimine. Biotechnol Prog. 2000 Mar-Apr;16(2):254-7.
-OH.png)
(S)-3-(4-Aminophenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid, 4-Amino-N-(tert-butoxycarbonyl)-L-phenylalanine, C14H20N2O4 CAS NO.55533-24-9 280.324, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation: 25.6°(C=0.01g/mL, MEOH, 20°C, 589nm), mp124-126 °C, bp484.9°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-910.
-3-(4-Aminophenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid.png)
(2S)-2-[(tert-Butoxycarbonyl)amino]-3-(3-fluorophenyl)propionic acid, N-(tert-Butoxycarbonyl)-3-fluoro-L-phenylalanine, C14H18FNO4 CAS NO.114873-01-7 MW283.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay: ≥95.0%, Water(KF): 0.11%, mp75-80°C, bp425°C at 760 mmHg, Flash point 210.8°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-926.
-2-[(tert-Butoxycarbonyl)amino]-3-(3-fluorophenyl)propionic acid.png)
(S)-2-((tert-Butoxycarbonyl)amino)-3-(4-fluorophenyl)propanoic acid, N-(tert-Butoxycarbonyl)-4-fluoro-L-phenylalanine, C14H18FNO4 CAS NO.41153-30-4 MW283.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.53%, Optical Rotation: 8.8°(C=1.00g/100ml MEOH), mp80°C, bp431.2°C at 760 mmHg, Flash point 214.6°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-888.
-2-((tert-Butoxycarbonyl)amino)-3-(4-fluorophenyl)propanoic acid.png)
Z-D-Phg-OH, N-Carbobenzoxy-D-2-phenylglycine, Cbz-D-(-)-Phenylglycine, C16H15NO4 CAS NO.17609-52-8 MW285.29, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.82%, Water(KF): 0.35%, OR[α](C=1.0 g/100ml ETOH): -113.4°, mp130-135 °C, bp495.3±45.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Frycák P, et al. High throughput multiplexed method for evaluation of enantioselective performance of chiral selectors by HPLC-ESI-MS and dynamic titration: cinchona alkaloid carbamates discriminating N-blocked amino acids. Chirality. 2009 Nov;21(10):929-36.

2-((tert-Butoxycarbonyl)amino)-2-(4-chlorophenyl)acetic acid, C13H16ClNO4 CAS NO.209525-73-5 MW285.72, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.73%, bp438.8±40.0 °C at 760 mmHg, mp101-103°C, bp429°C at 760 mmHg, Storage: Storage temp. 2-8°C
amino)-2-(4-chlorophenyl)acetic acid.png)
(S)-4-(tert-Butoxy)-3-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid, 1-tert-Butyl N-(tert-Butoxycarbonyl)-L-aspartate, N-tert-Boc-L-aspartic Acid tert-Butyl Ester, C13H23NO6 CAS NO.34582-32-6 MW289.32, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml MEOH): -26.6°, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-4-(tert-Butoxy)-3-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid.png)
(R)-2-((tert-Butoxycarbonyl)amino)-3-(4-cyanophenyl)propanoic acid, N-tert-Butoxycarbonyl-4-cyanophenyl-D-alanine, C15H18N2O4 CAS NO.146727-62-0 MW290.31, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.61%, mp152.6°C, bp494°C at 760 mmHg, Storage: Store at room temperature
-2-((tert-Butoxycarbonyl)amino)-3-(4-cyanophenyl)propanoic acid.png)
H-Phe(4-I)-OH, 4-Iodo-L-phenylalanine, (S)-2-Amino-3-(4-iodophenyl)propanoic Acid, C9H10INO2 CAS NO.24250-85-9 MW291.09, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Enantiomeric Excess: 100%, Assay (NMR): ≥98.0%, Water(KF): 0.73%, mp255-256 °C, bp366.8°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-822. [2]. Charles E Melan?on rd, et al. One plasmid selection system for the rapid evolution of aminoacyl-tRNA synthetases. Bioorg Med Chem Lett. 2009 Jul 15;19(14):3845-7. [3]. D Hellwig, et al. Para-[(123)I]iodo-L-phenylalanine in patients with pancreatic adenocarcinoma: tumour uptake, whole-body kinetics, dosimetry. Nuklearmedizin. 2008;47(5):220-4. [4]. D R Harding, et al. Synthesis of peptide labelled with p-iodophenylalanine which corresponds to the first amphipathic region of apolipoprotein C-I. Int J Pept Protein Res. 1985 Aug;26(2):208-13. [5]. Dirk Hellwig, et al. Validation of brain tumour imaging with p-[123I]iodo-L-phenylalanine and SPECT. Eur J Nucl Med Mol Imaging. 2005 Sep;32(9):1041-9.
-OH.png)
H-Phe(4-Br)-Ome.HCl, Methyl 4-bromo-l-phenylalaninate HCl, C10H13BrClNO2 CAS NO.99359-32-7 MW294.57, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.62%, Optical Rotation[a]: 27.52°(C=0.010101 g/ml ETOH), Storage: Store at room temperature
-Ome.HCl.png)
Boc-Tyr(Me)-OH, N-α-t.-Boc-O-methyl-L-tyrosine, N-α-t.-Boc-p-methoxy-L-phenylalanine, N-(tert-butoxycarbonyl)-4-methoxyphenylalanine, C15H21NO5 CAS NO.53267-93-9 MW295.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 96.81%, Water(KF): 0.22%, bp462°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
Boc-L-Tyrosine methyl ester, C15H21NO5 CAS NO.4326-36-7 MW295.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.53%, mp100-104°C, bp452.7°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. [2]. Jie Zeng, et al. A Supramolecular Gel Approach to Minimize the Neural Cell Damage during Cryopreservation Process. Macromol Biosci. 2016 Mar;16(3):363-70.

H-Glu(OtBu)-OtBu (hydrochloride), C13H26ClNO4 CAS NO.32677-01-3 MW295.80, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp60-75°C, Storage: 2-8°C, sealed storage, away from moisture, [1]. Manolopoulou A, et al. Synthesis of potent antagonists of substance P by modifying the methionyl and glutaminyl residues of its C-terminal hexapeptide and without using D-amino acids. Int J Pept Protein Res. 1993 Apr;41(4):411-4. [2]. A Manolopoulou, et al. Synthesis of potent antagonists of substance P by modifying the methionyl and glutaminyl residues of its C-terminal hexapeptide and without using D-amino acids. Int J Pept Protein Res. 1993 Apr;41(4):411-4. [3]. M Antoniou, et al. Synthesis and biological activity of analogues of the C-terminal hexapeptide of substance P with modifications at glutaminyl and methioninyl residues. Structure-activity studies. Int J Pept Protein Res. 1992 Nov;40(5):395-400.
-OtBu (hydrochloride).png)
Boc-Glu(OtBu)-OH, N-α-t.-Boc-L-glutamic acid γ-t.-butyl ester, C14H25NO6 CAS NO.13726-84-6 MW303.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%Optical Rotation[a]: -9.5°(C=0.01 g/mL MeOH), mp102-105°C, bp449.8°C at 760 mmHg, Storage: Storage temp. 2-8°C
-OH.png)
Boc-Glu-OtBu, Boc-L-glutamic acid 1-tert-butyl ester, (4R)-5-tert-butoxy-4-[(tert-butoxycarbonyl)amino]-5-oxopentanoic acid, C14H25NO6 CAS NO.24277-39-2 MW303.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.2%, Optical Rotation: -30.8554° (C=1.0042 g/100ml, MEOH), mp111.0-115.0 °C, bp449.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. [2]. Wanyi Tai, et al. Folding graft copolymer with pendant drug segments for co-delivery of anticancer drugs. Biomaterials. 2014 Aug;35(25):7194-203.

Boc-Dap(Boc)-OH,2,3-bis((tert-butoxycarbonyl)amino)propanoic acid, C13H24N2O6 CAS NO.88971-40-8 MW304.34, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Store at room temperature
-OH.png)
H-Trp(Boc)-OH, N-Indole-T-Butoxycarbonyl-L-Tryptophan, 1-(tert-butoxycarbonyl)tryptophan, C16H20N2O4 CAS NO.146645-63-8 MW304.34, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.22%, bp476.7±55.0 °C at 760 mmHg, Storage: Store at room temperature
-OH.png)
Z-Val-Gly-OH,[((2S)-2-{[(benzyloxy)carbonyl]amino}-3-methylbutanoyl)amino]acetic acid, C15H20N2O5 CAS NO.2790-84-3 MW308.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.12%, Optical Rotation: -25.73°(C=0.90g/100ml MEOH), Storage: Storage temp. 2-8°C, [1]. Kawashiro K, et al. Esterification of N-benzyloxycarbonyldipeptides in ethanol-water with immobilized papain. Biotechnol Bioeng. 1993 Jul;42(3):309-14.

Fmoc-Sar-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N-methylglycine, Fmoc-N-methylglycine, Fmoc-sarcosine, [[(9H-fluoren-9-ylmethoxy)carbonyl](methyl)amino]acetic acid, C18H17NO4 CAS NO.77128-70-2 MW311.34, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.76%, mp117.0-121.0 °C, bp512.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. [2]. Ewa Radzikowska, et al. Synthesis of PS/PO-chimeric oligonucleotides using mixed oxathiaphospholane and phosphoramidite chemistry. Org Biomol Chem. 2015 Jan 7;13(1):269-76.
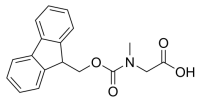
H-Asp(Obzl)-OtBu.HCl, 4-benzyl 1-(tert-butyl) aspartate hydrochloride, C15H22ClNO4 CAS NO.52615-97-1 MW315.79, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: 2-8°C, sealed storage, away from moisture, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OtBu.HCl.png)
Boc-Orn(Aloc)-OH, 5-(((allyloxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)pentanoic acid, C14H24N2O6 CAS NO.171820-74-9 MW316.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp128-132 °C, bp504.9 °C at 760 mmHg, Storage: Store at room temperature
-OH.png)
(tert-Butoxycarbonyl)-L-valyl-L-valine, (tert-butoxycarbonyl)valylvaline, C15H28N2O5 CAS NO.69209-73-0 MW316.39, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-L-valyl-L-valine.png)
(R)-2-((tert-Butoxycarbonyl)amino)-3-(2,4,5-trifluorophenyl)propanoic acid, C14H16F3NO4 CAS NO.486460-09-7 MW319.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Store at room temperature
-2-((tert-Butoxycarbonyl)amino)-3-(2,4,5-trifluorophenyl)propanoic acid.png)
((Benzyloxy)carbonyl)-L-alanyl-L-valine, C16H22N2O5 CAS NO.14550-79-9 MW322.36, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥96.0%Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
carbonyl)-L-alanyl-L-valine.png)
Boc-Phe-Gly-OH, ({2-[(tert-butoxycarbonyl)amino]-3-phenylpropanoyl}amino)acetic acid, C16H22N2O5 CAS NO.25616-33-5 MW322.36, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml DMF): -11.0°, mp163-164 °C, bp588.5±50.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Jun-Liang Zeng, et al. Generating Monofluoro-Substituted Amines and Amino Acids by the Interaction of Inexpensive KF and Sulfamidates. Chemistry Europe. Volume 2022, Issue 17, May 6, 2022
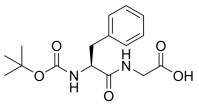
Fmoc-Aib-OH, 2-[(9H-Fluoren-9-ylmethoxy)carbonylamino]isobutyric Acid, C19H19NO4 CAS NO.94744-50-0 MW325.38, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.90%, Residue on Ignition: 0.01%, mp182-188°C, bp544.3°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1065.

Fmoc-N-Me-D-Ala-OH, N-α-Fmoc-N-α-methyl-D-alanine, C19H19NO4 CAS NO.138774-92-2 MW325.364, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.90%, Optical Rotation: 18.0521° (C=1.0021 g/100ml, DMF), bp514.9°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1061.

Methyl (R)-2-((tert-butoxycarbonyl)amino)-3-iodopropanoate, N-(tert-Butoxycarbonyl)-3-iodo-L-alanine Methyl Ester, C9H16INO4 CAS NO.93267-04-0 MW329.13, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (QNMR): 99.30%, Optical Rotation[a]: 41.6263°(C=0.5153g/100ml CHCL3), Water(KF): 0.01%, mp49.2-49.6°C, bp356.5°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1096.
-2-((tert-butoxycarbonyl)amino)-3-iodopropanoate.png)
(S)-2-[(tert-Butoxycarbonyl)amino]-3-iodopropionic acid methyl ester, Methyl (2S)-2-(tert-butoxycarbonylamino)-3-iodopropanoate; Methyl (S)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate, C9H16INO4 CAS NO.170848-34-7 MW329.13, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=0.75450 MEOH): 4.2°, mp55-59°C, bp356.5°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-2-[(tert-Butoxycarbonyl)amino]-3-iodopropionic acid methyl ester.png)
Fmoc-Pra-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-propargylglycine, C20H17NO4 CAS NO.198561-07-8 MW335.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Enantiomeric Excess: 99.98%, Assay (HPLC): 99.90%, Water(KF): 0.08%, Optical Rotation: 8.4416°(C=1.0081g/100ml MEOH), mp175°C, bp576.7°C at 760 mmHg, Flash point 302.6°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. Fmoc-Pra-OH is a Fmoc-protected glycine derivative used in solid-phase peptide synthesis technology, this compound can be used as an unusual amino acid analog to help deconvolute protein structure and function. N-Fmoc-L-propargylglycine is non-natural amino acid used for triazole crosslinking.

(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-cyanopropanoic acid, Fmoc-beta-cyano-L-alanine, C19H16N2O4 CAS NO.127273-06-7 MW336.34, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=0.98 g/100ml DMF): -38.4°, mp106-107 °C, bp617.1±55.0 °C at 760 mmHg, Storage: Store at room temperature
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-cyanopropanoic acid.png)
(S)-2-(tert-butoxycarbonylamino)-3-(4-carbamoyl-2,6-dimethylphenyl)propanoic acid, C17H24N2O5 CAS NO.623950-02-7 MW336.388, form Solid, color White to off-white, Assay (HPLC): 99.71%, mp>210°C, bp497.1±45.0°C at 760 mmHg, Flash point 254.4±28.7°C, Storage: Store at room temperature, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-839.
-2-(tert-butoxycarbonylamino)-3-(4-carbamoyl-2,6-dimethylphenyl)propanoic acid.png)
Fmoc-Gly(allyl)-OH, C20H19NO4 CAS NO.146549-21-5 MW337.37, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Enantiomeric Excess: 99.78% Assay (HPLC): 99.70%, Optical Rotation: -8.633° (C=0.01 g/ml, DMF), mp137.1°C, bp563°C at 760 mmHg, Flash point 294.3°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
Fmoc-D-Gly(allyl)-OH, C20H19NO4 CAS NO.170642-28-1 MW337.375, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Enantiomeric Excess: 99.56%, Assay (HPLC): 99.98%, mp134 °C, bp563 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)pentanoic acid, C20H21NO4 CAS NO.135112-28-6 MW339.39, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp151-153°C, bp557.9±33.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)pentanoic acid.png)
Glycyl-L-histidyl-L-lysine, C14H24N6O4 CAS NO.49557-75-7 MW340.38, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.60%, mp>144 °C, bp831.0±65.0 °C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Arkadiusz Gruchlik, et al. Effect of Gly-Gly-His, Gly-His-Lys and their copper complexes on TNF-alpha-dependent IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012 Nov-Dec;69(6):1303-6. [2]. I Bobyntsev, et al. Anxiolytic effects of Gly-His-Lys peptide and its analogs. Bull Exp Biol Med. 2015 Apr;158(6):726-8. [3]. M Yu Smakhtin, et al. Tripeptide Gly-His-Lys is a hepatotropic immunosuppressor. Bull Exp Biol Med. 2002 Jun;133(6):586-7.

(S)-3-(4-Bromophenyl)-3-((tert-butoxycarbonyl)amino)propanoic acid, C14H18BrNO4 CAS NO.261165-06-4 MW344.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Enantiomeric Excess: 100%, Purity (NMR): ≥98.0%, Optical Rotation: -46.00°(C=1.00g/100ml ETOH), mp143.8°C, bp479.1°C at 760 mmHg, Flash point 243.6°C, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-808.
-3-(4-Bromophenyl)-3-((tert-butoxycarbonyl)amino)propanoic acid.png)
(R)-3-(4-Bromophenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid, N-(tert-Butoxycarbonyl)-4-bromo-D-phenylalanine, C14H18BrNO4 CAS NO.79561-82-3 MW344.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.48%, Optical Rotation: -13.9 ° (C=0.011374 g/ml, MEOH), mp112.5°C, bp475.3°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-905.
-3-(4-Bromophenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid.png)
N2,N6-Bis(tert-butoxycarbonyl)-D-lysine, C16H30N2O6 CAS NO.65360-27-2 MW346.42, form Solid-liquid mixture, color Colorless to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥95.0%, bp514.4±45.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-D-lysine.png)
(R)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-cyclopropylpropanoic acid, C21H21NO4 CAS NO.170642-29-2 MW351.40, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.82%, mp155-157 °C, bp583.523 °C at 760 mmHg, Storage: Store at room temperature
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-cyclopropylpropanoic acid.png)
Fmoc-D-Leu-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-D-leucine, C21H23NO4 CAS NO.114360-54-2 MW353.41, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, mp155°C, bp559.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

Fmoc-N-Me-Val-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N-methyl-L-valine, C21H23NO4 CAS NO.84000-11-3 MW353.41, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.77%, Water(KF): 0.03%, Optical Rotation[a]: -68.035°(C=0.01 g/ml DMF), mp187-190°C, bp527.6±29.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Harris KS, et al. Rapid optimization of a peptide inhibitor of malaria parasite invasion by comprehensive N-methyl scanning. J Biol Chem. 2009 Apr 3;284(14):9361-71.

(S)-2-(((Benzyloxy)carbonyl)amino)-5-((tert-butoxycarbonyl)amino)pentanoic acid, N-a-CBZ-(N-d-BOC)-L-Orn, C18H26N2O6 CAS NO.7733-29-1 MW366.41, form Solid, color White to off-white, LCMS: Consistent with structure, Assay (LCMS): 99.02%, mp96-103°C, bp579.1±50.0°C at 760 mmHg, Storage: Storage temp. 2-8°C
-2-(((Benzyloxy)carbonyl)amino)-5-((tert-butoxycarbonyl)amino)pentanoic acid.png)
Boc-Orn(Z)-OH, Nα-(tert-Butoxycarbonyl)-Nδ-benzyloxycarbonyl-L-ornithine, C18H26N2O6 CAS NO.2480-93-5 MW366.41, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=1.01g/100ml CHCL3): 16.8°, Water(KF): 0.51%, mp98-104°C, bp579.1 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-OH.png)
4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)tetrahydro-2H-pyran-4-carboxylic acid, 4-(FMOC-AMINO)-TETRAHYDROPYRAN-4-CARBOXYLIC ACID, C21H21NO5 CAS NO.285996-72-7 MW367.40, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.77%, bp609.9±55.0 °C at 760 mmHg, Storage: Store at room temperature
methoxy)carbonyl)amino)tetrahydro-2H-pyran-4-carboxylic acid.png)
(R)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)hexanoic acid, FMoc-N-Methyl-D-norleucine, C22H25NO4 CAS NO.1217482-47-7 MW367.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 99.18%, Storage: Store at room temperature
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)hexanoic acid.png)
Fmoc-N-Me-Leu-OH, C22H25NO4 CAS NO.103478-62-2 MW367.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.23%, Water(KF): 0.14%, mp113-116°C, bp537.3°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Harris KS, et al. Rapid optimization of a peptide inhibitor of malaria parasite invasion by comprehensive N-methyl scanning. J Biol Chem. 2009 Apr 3;284(14):9361-71.
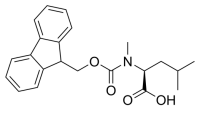
Fmoc-N-Me-Ile-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N-methyl-L-isoleucine, C22H25NO4 CAS NO.138775-22-1 MW367.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.91%, mp177-183°C, bp537.3°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Harris KS, et al. Rapid optimization of a peptide inhibitor of malaria parasite invasion by comprehensive N-methyl scanning. J Biol Chem. 2009 Apr 3;284(14):9361-71.
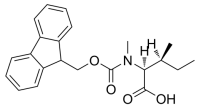
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-5-methyl-L-norleucine, C22H25NO4 CAS NO.180414-94-2 MW367.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.32%, bp568.1°C at 760 mmHgStorage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
carbonyl]-5-methyl-L-norleucine.png)
N-9-Fluorenylmethoxycarbonylaspartic acid β-methyl ester, Fmoc-Asp(OMe)-OH, C20H19NO6 CAS NO.145038-53-5 MW369.37, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.86%, OR[α](C=1.03 g/100ml CHCL3): 37.6°, Water(KF): 0.74%, mp122-125 °C, bp613.7±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
H-Lys(Z)-OtBu.HCl, H-Lys(z)-OtBu Hydrochloride, C18H29ClN2O4 CAS NO.5978-22-3 MW372.89, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.44%, mp144-145 °C, bp469.6°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OtBu.HCl.png)
Fmoc-Chg-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-cyclohexylglycine, C23H25NO4 CAS NO.161321-36-4 MW379.45, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.52%, bp602.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.

Boc-Tyr(Boc)-OH, C19H27NO7 CAS NO.20866-48-2 MW381.42, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 98.36%, mp94-97 °C, bp528.4±50.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Osaka K, et al. Sequential Intermolecular Radical Addition and Reductive Radical Cyclization of Tyrosine and Phenylalanine Derivatives with Alkenes via Photoinduced Decarboxylation: Access to Ring-Constrained γ-Amino Acids. J Org Chem. 2019 Aug 2;84(15):9480-9488.
-OH.png)
Fmoc-Ala-Ala-OH, (((9H-Fluoren-9-yl)methoxy)carbonyl)-L-alanyl-L-alanine, C21H22N2O5 CAS NO.87512-31-0 MW382.41, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.49%, Water(KF): 0.26%, OR(C=1.00g/100ml MeOH): -29.1°, mp175-179 °C, bp674.2±45.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. V. Jayawarna, et al. Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides[J]. Advanced materials.2006 Mar 02

(2S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-(methylsulfinyl)butanoic acid, Fmoc-Met(O)-OH, C20H21NO5S CAS NO.76265-70-8 MW387.45, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Purity (NMR): ≥98.0%, bp698.0±55.0 °C at 760 mmHg, Storage: 2-8°C, stored under nitrogen
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-(methylsulfinyl)butanoic acid.png)
Fmoc-4-Pal-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-3-(4-pyridyl)-L-alanine, C23H20N2O4 CAS NO.169555-95-7 MW388.42, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Enantiomeric Excess: 100.00%, Assay (HPLC): 98.97%, Optical Rotation: -46.0° (C=1.00 g/100ml, DMF), mp219°C, bp646.9°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1038.

N-[N-[(1,1-Dimethylethoxy)carbonyl]-2-methylalanyl]-D-tryptophan, C20H27N3O5 CAS NO.159634-94-3 MW389.45, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Loss On Drying: 0.14%, Residue on Ignition: 0.04%, Water: 0.16%, mp165.5-165.7°C, Storage: Storage temp. 2-8°C
carbonyl]-2-methylalanyl]-D-tryptophan.png)
(S)-2-((tert-Butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoic acid, L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, C20H30BNO6 CAS NO.216439-76-8 MW391.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, bp537.2±50.0 °C at 760 mmHg, Storage: Store at room temperature
-2-((tert-Butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoic acid.png)
(S)-3-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-(allyloxy)-4-oxobutanoic acid, 1-Allyl N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-aspartate, C22H21NO6 CAS NO.144120-53-6 MW395.41, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, mp117-139°C, bp634.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-3-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-(allyloxy)-4-oxobutanoic acid.png)
H-His(Trt)-OH, C25H23N3O2 CAS NO.35146-32-8 MW397.48, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.90%, Water(KF): 0.15%, mp210°C, bp584.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
Fmoc-N-Me-Phe-OH, N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-N-methyl-L-phenylalanine, C25H23NO4 CAS NO.77128-73-5 MW401.46, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp143°C, bp595.4°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Harris KS, et al. Rapid optimization of a peptide inhibitor of malaria parasite invasion by comprehensive N-methyl scanning. J Biol Chem. 2009 Apr 3;284(14):9361-71.

(R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-aminophenyl)propanoic acid, Fmoc-4-amino-D-phenylalanine, C24H22N2O4 CAS NO.324017-21-2 MW402.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 97.56%, bp671.5±55.0°C at 760 mmHg, Storage: Store at room temperature
-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-aminophenyl)propanoic acid.png)
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-(methylsulfonyl)butanoic acid, Fmoc-met(O2)-OH, C20H21NO6S CAS NO.163437-14-7 MW403.45, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.96%, bp709.5±60.0 °C at 760 mmHg, Storage: Store at room temperature
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-(methylsulfonyl)butanoic acid.png)
Boc-Trp(Boc)-OH, BOC-N-IN-BOC-L-TRYPTOPHAN, C21H28N2O6 CAS NO.144599-95-1 MW404.46, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.85%, Optical Rotation[a]: -9.7°(C=0.01 g/ml DMF), mp160-161°C, Storage: Store at room temperature
-OH.png)
Fmoc-D-Lys-OH.HCl, Nα-[(9H-Fluoren-9-ylmethoxy)carbonyl]-D-lysine Hydrochloride, C21H25ClN2O4 CAS NO.201002-47-3 MW404.89, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Enantiomeric Excess: 100.00%, Assay (HPLC): 97.98%, Storage: 2-8°C, sealed storage, away from moisture, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
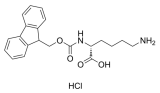
Z-Phe-Leu-OH, N-CBZ-Phe-Leu, CBZ-phenylalanylleucine, C23H28N2O5 CAS NO.4313-73-9 MW412.48, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, bp674.4°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Makino M, et, al. Carboxypeptidase Y activity and maintenance is modulated by a large helical structure. FEBS Open Bio. 2019 Jul;9(7):1337-1343. [2]. H Ostrowska, et al. Cathepsin A-like activity is possibly the main acidic carboxypeptidase in human platelets. Platelets. 1997;8(5):355-60. [3]. Joji Mima, et al. Amphipathic property of free thiol group contributes to an increase in the catalytic efficiency of carboxypeptidase Y. Eur J Biochem. 2002 Jul;269(13):3220-5. [4]. Li Li, et al. Debittering effect of Actinomucor elegans peptidases on soybean protein hydrolysates. J Ind Microbiol Biotechnol. 2008 Jan;35(1):41-7. [5]. Long-Liu Lin, et al. Characterization of a bifunctional aminoacylase/carboxypeptidase from radioresistant bacterium Deinococcus radiodurans R1. J Biotechnol. 2007 Feb 1;128(2):322-34.
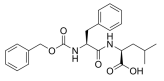
Fmoc-Phe(4-Cl)-OH, C24H20ClNO4 CAS NO.175453-08-4 MW421.87, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.76%, mp138 °C, bp640.9 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-889.
-OH.png)
(R)-3-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)propanoic acid, Boc-D-Dap(Fmoc)-OH, C23H26N2O6 CAS NO.131570-56-4 MW426.46, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, bp652.5°C at 760 mmHg, Storage: Store at room temperature
-3-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)propanoic acid.png)
H-Arg(Pbf)-OH,(E)-N~5~-(amino{[(2,2,4,6,7-pentamethyl-2,3-dihydro-1-benzofuran-5-yl)sulfonyl]amino}methylidene)-L-ornithine, 2-amino-5-{N'-[(2,2,4,6,7-pentamethyl-3H-1-benzofuran-5-yl)sulfonyl]carbamimidamido}pentanoic acid, C19H30N4O5S CAS NO.200115-86-2 MW426.53, form Solid, color White to off-white, Assay (NMR): ≥98.0%, bp636.9°C at 760 mmHg, Storage: Store at room temperature
-OH.png)
Fmoc-amino-PEG3-CH2COOH, 1-(9H-fluoren-9-yl)-3-oxo-2,7,10,13-tetraoxa-4-azapentadecan-15-oic acid, C23H27NO7 CAS NO.139338-72-0 MW429.46, form <46°C Solid,>46°C Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.60%, mp46°C, bp658.9±50.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. An S, et al. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 2018 Oct;36:553-562

(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(3-chloro-4-hydroxyphenyl)propanoic acid, Fmoc-L-3-Chlorotyrosine, C24H20ClNO5 CAS NO.478183-58-3 MW437.87, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.36%, Optical Rotation[a]: -20.5°(C=0.01g/ml DMF), bp674.9°C at 760 mmHg, Flash point 362°C, Storage: Store at room temperature
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(3-chloro-4-hydroxyphenyl)propanoic acid.png)
Fmoc-N-Me-Glu(OtBu)-OH, 2-((((9H-fluoren-9-yl)methoxy)carbonyl)(methyl)amino)-5-(tert-butoxy)-5-oxopentanoic acid, C25H29NO6 CAS NO.200616-40-6 MW439.50, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.67%, mp16-119°C, bp612.3°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
(R)-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid, Boc-D-Dab(Fmoc)-OH, C24H28N2O6 CAS NO.131570-57-5 MW440.49, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Enantiomeric Excess: 99.20%, Assay (NMR): ≥97.0%, OR[α](C=1.00 g/100ml MEOH): 10.7°, bp670.9±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid.png)
N-(((9H-Fluoren-9-yl)methoxy)carbonyl)-O-benzyl-N-methyl-L-threonine, C27H27NO5 CAS NO.198561-81-8 MW445.51, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 95.88%, mp116-122 °C, bp622.0±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
methoxy)carbonyl)-O-benzyl-N-methyl-L-threonine.png)
Fmoc-Orn(Boc)-OH, Nδ-(tert-Butoxycarbonyl)-Nα-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-ornithine, C25H30N2O6 CAS NO.109425-55-0 MW454.52, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 98.96%, mp111-115°C, bp679°C at 760 mmHg, Storage: Storage temp. 2-8°C
-OH.png)
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(2,4-dichlorophenyl)propanoic acid, 2,4-dichloro-N-[(9H-fluoren-9-ylmethoxy)carbonyl]phenylalanine, C24H19Cl2NO4 CAS NO.352351-62-3 MW456.32, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.18%, Optical Rotation[a]: -45.9°(C=0.01g/ml DMF), bp659.9±55.0 °C at 760 mmHg, Storage: Store at room temperature
-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(2,4-dichlorophenyl)propanoic acid.png)
Fmoc-Bip(4,4')-OH, 2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-(biphenyl-4-yl)propanoic acid, C30H25NO4 CAS NO.199110-64-0 MW463.52, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 97.95%, OR(C=1.00g/100ml DMF): -11.5°, bp700.7°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1073.
-OH.png)
Fmoc-His(Boc)-OH, 2-((((9H-fluoren-9-yl)methoxy)(hydroxy)methylene)amino)-3-(1-(tert-butoxycarbonyl)-1H-imidazol-4-yl)propanoic acid, C26H27N3O6 CAS NO.81379-52-4 MW477.51, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Enantiomeric Excess: 98.17%, Assay (HPLC): 97.64%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
Fmoc-D-Bpa-OH, C31H25NO5 CAS NO.117666-97-4 MW491.53, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structurePurity (HPLC): 98.31%, bp729.037 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-960.

Boc-His(Trt)-OH, Nα-(tert-butoxycarbonyl)-Nt-tritylhistidine, Nα-Boc-N(im)-trityl-L-histidine, C30H31N3O4 CAS NO.32926-43-5 MW497.58, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=1.00 g/100ml MEOH): 11.6°, mp130°C, bp667.5°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144. [2]. Ngoc-Duc Doan, et al. Solid-phase synthesis of C-terminal azapeptides. J Pept Sci. 2015 May;21(5):387-91.
-OH.png)
Fmoc-L-Lys(Dde)-OH, N~6~-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl]-N~2~-[(9H-fluoren-9-ylmethoxy)carbonyl]lysine, Nα-[(9H-Fluoren-9-ylmethyloxy)carbonyl]-Nε-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl-L-lysine, C31H36N2O6 CAS NO.150629-67-7 MW532.63, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.30%, Water(KF): 0.05%, Optical Rotation: 2.6° (C=0.01 g/ml, MEOH), mp80°C, bp750.1±60.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
Methyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-hydroxy-3,5-diiodophenyl)propanoate, Boc-3,5-diiodo-tyr-ome, C15H19I2NO5 CAS NO.128781-80-6 MW547.12, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp126-128 °C, bp494.5±45.0 °C at 760 mmHg, Storage: 2-8°C, protect from light
-2-((tert-butoxycarbonyl)amino)-3-(4-hydroxy-3,5-diiodophenyl)propanoate.png)
Fmoc-L-Lys(ivDde)-OH, N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N6-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)-3-methylbutyl)lysine, Nα-Fmoc-Nε-[1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl]-L-lysine, C34H42N2O6 CAS NO.204777-78-6 MW574.71, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.30%, Optical Rotation: -5.2°(C=1g/100ml DMF), bp 765.6°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
(S)-methyl 2-((S)-4-methyl-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamido)-3-phenylpropanoate, C32H44N4O6 CAS NO.1140908-89-9 MW580.72, form Solid, color White to off-white, Assay: 97.18%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-865.
-methyl 2-((S)-4-methyl-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamido)-3-phenylpropanoate.png)
N2,N6-Bis(((9H-fluoren-9-yl)methoxy)carbonyl)-D-lysine, C36H34N2O6 CAS NO.75932-02-4 MW590.66, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
methoxy)carbonyl)-D-lysine.png)
Fmoc-D-Asn(Trt)-OH, Fmoc-N-trityl-D-asparagine, C38H32N2O5 CAS NO.180570-71-2 MW596.67, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.80%, mp211-216°C, bp831.4°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Luckose F, et al. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit Rev Food Sci Nutr. 2015;55(13):1793-1144.
-OH.png)
4',5'-Didehydro-5'-deoxyuridine, 1-(5-Deoxy-beta-D-erythro-pent-4-enofuranosyl)uracil, 1-(3,4-dihydroxy-5-methylideneoxolan-2-yl)-4-hydroxy-1,2-dihydropyrimidin-2-one, C9H10N2O5 CAS NO.14365-63-0 MW226.19, Assay 95%, mp169-170°C, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.
2,2'-Anhydrouridine, 2,2'-Cyclouridine, O2,2'-Cyclouridine, C9H10N2O5 CAS NO.3736-77-4 MW226.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay ≥98.0%, mp239-240°C, bp456.3°C at 760 mmHg, Storage: Storage temp. -20°C
2'-Deoxycytidine, Deoxycytidine, Cytosine deoxyriboside, Deoxyribose cytidine, C9H13N3O4 CAS NO.951-77-9 MW227.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 99.66%, mp209-211°C, bp497.6°C at 760 mmHg, Flash point 254.8°C, Storage: 2-8°C, protect from light, [1]. Horn D, et al. Inhibition of biological effects of bromodeoxyuridine by deoxycytidine: correlation with decreased incorporation of bromodeoxyuridine into DNA. Somatic Cell Genet. 1976 Sep;2(5):469-81. [2]. Akihiko Hatano, et al. Kinetic parameters and recognition of thymidine analogues with varying functional groups by thymidine phosphorylase. Bioorg Med Chem. 2008 Apr 1;16(7):3866-70. [3]. Charlotte Stentoft, et al. Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2014 Aug 22:1356:197-210. [4]. Claudia Fiorini, et al. Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. Biochim Biophys Acta. 2015 Jan;1853(1):89-100. [5]. Dominique Thomas, et al. Quantitation of endogenous nucleoside triphosphates and nucleosides in human cells by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2015 May;407(13):3693-704.

1-((2R,6S)-6-(Hydroxymethyl)morpholin-2-yl)pyrimidine-2,4(1H,3H)-dione, 1-[(2R,6S)-6-(hydroxyMethyl)-2-Morpholinyl]-2,4(1H,3H)-PyriMidinedione, C9H13N3O4 CAS NO.109205-43-8 MW227.22, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.30%, Storage: 2-8°C, stored under nitrogen
-6-(Hydroxymethyl)morpholin-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
2'-Deoxyuridine, C9H12N2O5 CAS NO.951-78-0 MW228.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.97%, mp167-169°C, bp370.01 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Reidy JA, et al. Deoxyuridine increases folate-sensitive fragile site expression in human lymphocytes. Am J Med Genet. 1987 Jan;26(1):1-5. [2]. Akihiko Hatano, et al. Kinetic parameters and recognition of thymidine analogues with varying functional groups by thymidine phosphorylase. Bioorg Med Chem. 2008 Apr 1;16(7):3866-70. [3]. Charlotte Stentoft, et al. Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2014 Aug 22:1356:197-210. [4]. Dorota Rybaczek, et al. Caffeine-Induced Premature Chromosome Condensation Results in the Apoptosis-Like Programmed Cell Death in Root Meristems of Vicia faba. PLoS One. 2015 Nov 6;10(11):e0142307. [5]. Ilaria Naldi, et al. Novel epigenetic target therapy for prostate cancer: a preclinical study. PLoS One. 2014 May 22;9(5):e98101.

Decitabine, 5-Aza-2'-deoxycytidine, 5-AZA-CdR, C8H12N4O4 CAS NO.2353-33-5 MW228.21, form Solid, color White to off-white, LCMS: Consistent with structure, Assay (LCMS): 99.74%, mp200°C, bp485.8°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Hagemann S, et al. Azacytidine and decitabine induce gene-specific and non-random DNA demethylation in human cancer cell lines. PLoS One. 2011 Mar 7;6(3):e17388. [2]. Nakamura M, et al. Decitabine inhibits tumor cell proliferation and up-regulates E-cadherin expression in Epstein-Barr virus-associated gastric cancer. J Med Virol. 2016 Jul 19. [3]. Parker WB. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem Rev. 2009 Jul;109(7):2880-93. [4]. Requena CE, et al. The nucleotidohydrolases DCTPP1 and dUTPase are involved in the cellular response to decitabine. Biochem J. 2016 Jun 20. [5]. Terse P, et al. Subchronic oral toxicity study of decitabine in combination with tetrahydrouridine in CD-1 mice. Int J Toxicol. 2014 Mar-Apr;33(2):75-85.
2',3'-Dideoxyadenosine(Didanosine Impurity), CAS NO.4097-22-7, Assay 98%, mp181-184 °C, bp377.64 °C at 760 mmHg, Storage : 2-8°C, protect from light, [1]. Amit Singhal, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014 Nov 19;6(263):263ra159. [2]. Andrew C Emery, et al. A new site and mechanism of action for the widely used adenylate cyclase inhibitor SQ22,536. Mol Pharmacol. 2013 Jan;83(1):95-105. [3]. Cecilia Herraiz, et al. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol Endocrinol. 2011 Jan;25(1):138-56. [4]. Cesar M Rueda, et al. Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur J Immunol. 2015 Sep;45(9):2582-92. [5]. Christopher Tubbs, et al. Identification of 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor binding and membrane progestin receptor alpha on southern flounder sperm (Paralichthys lethostigma) and their likely role in 20β-S stimulation of sperm hypermotility. Gen Comp Endocrinol. 2011 Feb 1;170(3):629-39.
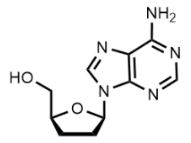
5-Methyl-2'-deoxycytidine, 5-Methyldeoxycytidine, C10H15N3O4 CAS NO.838-07-3 MW241.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation[a]: 41.1°(C=0.009986 g/ml H2O), mp217-219°C, bp492.8°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Christman JK, et al. 5-Methyl-2'-deoxycytidine in single-stranded DNA can act in cis to signal de novo DNA methylation. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7347-51. [2]. Testillano PS, et al. The 5-methyl-deoxy-cytidine (5mdC) localization to reveal in situ the dynamics of DNA methylation chromatin pattern in a variety of plant organ and tissue cells during development. Physiol Plant. 2013 Sep;149(1):104-13.
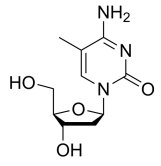
Thymidine, C10H14N2O5 CAS NO.50-89-5 MW242.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 99.76%, Water(KF): 0.10%, mp186-188°C, bp385.05 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Chen G, et al. Cell Synchronization by Double Thymidine Block. Bio Protoc. 2018 Sep 5;8(17). [2]. FIRKET H, et al. Autoradiographic visualization of synthesis of deoxyribonucleic acid in tissue culture with tritium-labelled thymidine. Nature. 1958 Jan 24;181(4604):274-5.FIRKET H, et al. Autoradiographic visualization of synthesis of deoxyribonucleic acid in tissue culture with tritium-labelled thymidine. Nature. 1958 Jan 24;181(4604):274-5. [3]. Izeradjene K, et al. Inhibition of thymidine synthesis by folate analogues induces a Fas-Fas ligand-independentdeletion of superantigen-reactive peripheral T cells. Int Immunol. 2001 Jan;13(1):85-93. [4]. David H Bechtel, et al. Genotoxicity testing of esterified propoxylated glycerol (EPG). Regul Toxicol Pharmacol. 2014 Dec:70 Suppl 2:S131-42. [5]. Hong-Bo Wang, et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat Commun. 2015 Feb 11:6:6240.
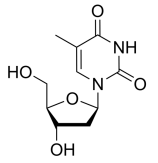
Cytidine, Cytosine β-D-riboside, Cytosine-1-β-D-ribofuranoside, C9H13N3O5 CAS NO.65-46-3, form Solid, color White to off-white, LCMS: Consistent with structure, Assay (LCMS): 98.78%, mp210-217°C, bp545.7°C at 760 mmHg, Flash point 283.8°C, Storage: Storage temp. 2-8°C, [1]. Jonas DA, et al. Safety considerations of DNA in food. Ann Nutr Metab. 2001;45(6):235-54. [2]. Machado-Vieira R, et, al. New therapeutic targets for mood disorders. ScientificWorldJournal. 2010 Apr 13;10:713-26. [3]. Wurtman RJ, et al. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000 Oct 1;60(7):989-92. [4]. Arun Sreekumar, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [5]. Carina Villacrés, et al. Low glucose depletes glycan precursors, reduces site occupancy and galactosylation of a monoclonal antibody in CHO cell culture. Biotechnol J. 2015 Jul;10(7):1051-66.

Ribavirin, C8H12N4O5 CAS NO.36791-04-5 MW244.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp174-176°C, bp639.8°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Abdel-Hamid NM, et al. Synergistic Effects of Jerusalem Artichoke in Combination with Pegylated Interferon Alfa-2a and Ribavirin Against Hepatic Fibrosis in Rats. Asian Pac J Cancer Prev. 2016;17(4):1979-85. [2]. Chang J, et al. Combination of α-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res. 2011 Jan;89(1):26-34 [3]. Refaat B, et al. The effects of pegylated interferon-α and ribavirin on liver and serum concentrations of activin-A and follistatin in normal Wistar rat: a preliminary report. BMC Res Notes. 2015 Jun 26;8:265 [4]. Robert O Baker, et al. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003 Jan;57(1-2):13-23. [5]. Savic D, et al. Ribavirin shows immunomodulatory effects on activated microglia. Immunopharmacol Immunotoxicol. 2014 Dec;36(6):433-41

5-Azacytidine, Ladakamycin, C8H12N4O5 CAS NO.320-67-2 MW244.20, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.35%, mp228-230°C, bp534.5°C at 760 mmHg, Flash point 277°C, Storage: Storage temp. 2-8°C, [1]. Christman JK. 5-Azacytidine and 5-aza-2'-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002 Aug 12;21(35):5483-95. [2]. Creusot F, et al. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidineand 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041-8. [3]. Li LH,et al. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970 Nov;30(11):2760-9. [4]. Marycz K, et al. 5-Azacytidine and Resveratrol Enhance Chondrogenic Differentiation of Metabolic Syndrome-Derived Mesenchymal Stem Cells by Modulating Autophagy.Oxid Med Cell Longev. 2019 May 12;2019:1523140. [5]. Denis Fourches, et al. Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species. Chem Res Toxicol. 2010 Jan;23(1):171-83.

1-beta-D-Arabinofuranosyluracil, C9H12N2O6 CAS NO.3083-77-0 MW244.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml H2O): 117.9°, mp220-222°C, Storage: Storage temp. 2-8°C, [1]. Bertin MJ, et al. Spongosine production by a Vibrio harveyi strain associated with the sponge Tectitethya crypta. J Nat Prod. 2015 Mar 27;78(3):493-9. [2]. Müller WE, et al. Metabolism of 1-beta-D-arabinofuranosyluracil in mouse L5178Y cells. Cancer Res. 1979 Mar;39(3):1102-7. [3]. Hancheng Cai, et al. The improved syntheses of 5-substituted 2'-[18F]fluoro-2'-deoxy-arabinofuranosyluracil derivatives ([18F]FAU, [18F]FEAU, [18F]FFAU, [18F]FCAU, [18F]FBAU and [18F]FIAU) using a multistep one-pot strategy. Nucl Med Biol. 2011 Jul;38(5):659-66. [4]. Herbert Schott, et al. N?-[Alkyl-(hydroxyphosphono)phosphonate]-cytidine-new drugs covalently linking antimetabolites (5-FdU, araU or AZT) with bone-targeting bisphosphonates (alendronate or pamidronate). Bioorg Med Chem. 2011 Jun 1;19(11):3520-6. [5]. Jae-Jun Park, et al. Comparison of cell-labeling methods with 12?I-FIAU and ??Cu-PTSM for cell tracking using chronic myelogenous leukemia cells expressing HSV1-tk and firefly luciferase. Cancer Biother Radiopharm. 2012 Dec;27(10):719-28.
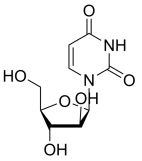
L-Uridine, C9H12N2O6 CAS NO.26287-69-4 MW244.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Storage temp. 2-8°C, [1]. A F Wu, et al. L-uridine: synthesis and behavior as enzyme substrate. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1222-6. [2]. Yumei Wang, et al. Chemical Composition and Pharmacology Research Progress of Shenlingbaizhu Powder. 2019 Asia-Pacific Conference on Clinical Medicine and Public Health.

Uridine, β-Uridine, C9H12N2O6 CAS NO.58-96-8 MW244.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 99.87%, mp163-167°C, bp387.12 °C at 760 mmHg, Storage: Store at room temperature, [1]. Eric A Lee, et al. Targeting Mitochondria with Avocatin B Induces Selective Leukemia Cell Death. Cancer Res. 2015 Jun 15;75(12):2478-88. [2]. Imen Lassoued, et al. Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Raja clavata) muscle. J Proteomics. 2015 Oct 14:128:458-68. [3]. M Polo, et al. Mitochondrial (dys)function - a factor underlying the variability of efavirenz-induced hepatotoxicity?. Br J Pharmacol. 2015 Apr;172(7):1713-27. [4]. S D Mesquita, et al. Lipocalin 2 modulates the cellular response to amyloid beta. Cell Death Differ. 2014 Oct;21(10):1588-99. [5]. Sang-Yong Lee, et al. Polyoxometalates--potent and selective ecto-nucleotidase inhibitors. Biochem Pharmacol. 2015 Jan 15;93(2):171-81.
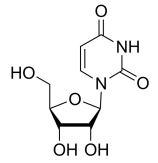
Alovudine, C10H13FN2O4 CAS NO.25526-93-6 MW244.22, form Solid, color hite to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.71%, mp176-178 °C, Storage: Storage temp. 2-8°C, Antiviral agents of potential use, which are active in a similar manner to other 3′-deoxy-3′-substituted thymid. [1]. Challapalli A, et al. 3'-Deoxy-3'-18F-fluorothymidine positron emission tomography as an early predictor of disease progression in patients with advanced and metastatic pancreatic cancer. Eur J Nucl Med Mol Imaging. 2015 May;42(6):831-40. [2]. Smee DF, et al. A review of compounds exhibiting anti-orthopoxvirus activity in animal models. Antiviral Res. 2003 Jan;57(1-2):41-52.

4-Amino-1-((2R,3R,4S,5R)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoropyrimidin-2(1H)-one (Capecitabine Impurity), 5'-Deoxy-5-fluorocytidine, C9H12FN3O4 CAS NO.66335-38-4 MW245.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.57%, mp196-198 °C, bp437.9±55.0 °C at 760 mmHg, Storage: 2-8°C, protect from light
-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoropyrimidin-2(1H)-one (Capecitabine Impurity).png)
5-Fluoro-2'-deoxycytidine, C9H12FN3O4 CAS NO.10356-76-0 MW245.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp196 °C, bp465 °C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Beumer JH, et, al. Pharmacokinetics, metabolism, and oral bioavailability of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine in mice. Clin Cancer Res. 2006 Dec 15;12(24):7483-91. [2]. Osterman DG, et, al. 5-Fluorocytosine in DNA is a mechanism-based inhibitor of HhaI methylase. Biochemistry. 1988 Jul 12;27(14):5204-10. [3]. D J Baker, et al. Transition state analogs as affinity labels for human DNA methyltransferases. Biochem Biophys Res Commun. 1993 Oct 29;196(2):864-71. [4]. Duoli Guo, et al. Stability of 5-fluoro-2'-deoxycytidine and tetrahydrouridine in combination. AAPS PharmSciTech. 2010 Mar;11(1):247-52. [5]. H Stopper, et al. Micronuclei induced by modulators of methylation: analogs of 5-azacytidine. Carcinogenesis. 1995 Jul;16(7):1647-50.

Floxuridine, 5-Fluorouracil 2'-deoxyriboside, C9H11FN2O5 CAS NO.50-91-9 MW246.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.76%, mp148°C, bp150 °C at 760 mmHg, Storage: Storage temp. 2-8°C, Applications Floxuridine can be used to target thymidylate synthase in targeted cancer chemotherapy. Experimental anticancer agents have shown activity a variety of malignant tumors, including mouse mammary tumors and colon-rectal cancer. [1]. Huehls AM, et al. Poly(ADP-Ribose) polymerase inhibition synergizes with 5-fluorodeoxyuridine but not 5-fluorouracil in ovarian cancer cells.Cancer Res. 2011 Jul 15;71(14):4944-54. [2]. Langman J, et al. Floxuridine and its influence on postnatal cerebellar development. Pediatr Res. 1972 Oct;6(10):758-64. [3]. Yeo WS, et al. The FDA-approved anti-cancer drugs, streptozotocin and floxuridine, reduce the virulence of Staphylococcus aureus.Sci Rep. 2018 Feb 6;8(1):2521. [4]. Alaa A Abd-Elsayed, et al. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain. 2015 Jul 31:11:45. [5]. Hua Du, et al. Reproductive Toxicity of Endosulfan: Implication From Germ Cell Apoptosis Modulated by Mitochondrial Dysfunction and Genotoxic Response Genes in Caenorhabditis elegans. Toxicol Sci. 2015 May;145(1):118-27.

Doxifluridine, 5-Fluoro-5'-deoxyuridine, C9H11FN2O5 CAS NO.3094-09-5 MW246.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (LCMS): 99.91%, mp188-192 °C, Storage: Storage temp. 2-8°C, Applications Doxifluridine is an antineoplastic agent effective in tumor, cell line, or fibroblast cell transformed by H-ras ork oncogene. It has antidyspepsive activity independent of its antiproliferative activity. [1]. Baek IH, Lee BY, Kim MS, Pharmacokinetic analysis of doxifluridine and its metabolites, 5-fluorouracil and 5-fluorouridine, after oral administration in beagle dogs. Eur J Drug Metab Pharmacokinet. 2013 Apr 7. [2]. Dipartimento di Chimica, Università degli Studi della Basilicata, Potenza, In vitro toxicity of N3-methyl-5'-deoxy-5-fluorouridine, a novel metabolite of doxifluridine: a bioanalytical investigation. J Pharm Biomed Anal. 1998 May;17(1):11-6. [3]. Yoshikawa T, Tsuburaya A, Shimada K, A phase II study of doxifluridine and docetaxel combination chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer. 2009;12(4):212-8. [4]. E Di Gennaro, et al. Vorinostat synergises with capecitabine through upregulation of thymidine phosphorylase. Br J Cancer. 2010 Nov 23;103(11):1680-91. [5]. Jelena Popovic, et al. Expression analysis of SOX14 during retinoic acid induced neural differentiation of embryonal carcinoma cells and assessment of the effect of its ectopic expression on SOXB members in HeLa cells. PLoS One. 2014 Mar 17;9(3):e91852.

2'-Deoxy-2'-fluorouridine, C9H11FN2O5 CAS NO.784-71-4 MW246.19, form Solid, color White to off-white, Assay: ≥98.0%, mp149-150°C, Storage: Storage temp. 2-8°C, Applications 2'-Fluoro-2'-deoxyuridine is as a component to be incorporated into therapeutic oligonucleotides for the treatment of prostate cancer and cancer. [1]. J V Tuttle, et al. Purine 2'-deoxy-2'-fluororibosides as antiinfluenza virus agents. J Med Chem
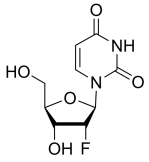
5,6-Dihydrouridine, C9H14N2O6 CAS NO.5627-05-4 MW246.22, form Solid, color White to off-white, LCMS: Consistent with structure, Assay (HPLC): 99.58%, Storage: 2-8°C, protect from light, Applications 5,6-Dihydrouridine (DHU) is a derivative of the pyrimidine nucleoside and uridine found in t from bacteria, archaea, and some eukaryotes, providing conformational flexibility. In an in vitro assay, 5,6-dihydrouridine inhibited herichia coli Uracil-DNA glycosylase, elevated levels of 5,6-dihydrouridine in the sera of prostate cancer positively correlate with lethality. [1]. Kasprzak JM, et al. Molecular evolution of dihydrouridine synthases. BMC Bioinformatics. 2012 Jun 28;13:153.
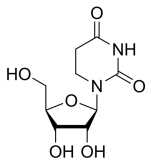
7-Deaza-2'-deoxyadenosine C11H14N4O3 CAS NO.60129-59-1 MW250.25, form Solid, color White to pink, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥97.0%, mp>217 °C, bp601 °C at 760 mmHg, Storage: 2-8°C, protect from light

2'-Deoxyadenosine, C10H13N5O3 CAS NO.958-09-8 MW251.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp187-189°C, bp394.4 °C at 760 mmHg, Storage: 2-8°C, protect from light, Applications 2'-Deoxyadenosine is for the study of anomalous stimulation of human sperm motility. It plays an important role in mouse and human embryonic development sperminjection as a sperm motility stimulant. It is applied as an energy source in some cells under energy stress conditions. It involves various biological processes to compare the functions of adine analogs.
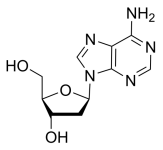
Cordycepin, 3'-Deoxyadenosine, C10H13N5O3 CAS NO.73-03-0 MW251.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥99.0%, mp225-229°C, bp627.2°C at 760 mmHg, Storage: Storage temp. -20°C, Applications Cordycepin is an adenosine analog that can be easily converted into cordycepin 5′-triphosphate; it can be for 3′-end labeling of RNA. [1]. Huang F, et al. Cordycepin kills Mycobacterium tuberculosis through hijacking the bacterial adenosine kinase. PLoS One. 2019 Jun 14;14(6):e0218449. [2]. Noh EM, et al. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford). 2009 Jan;48(1):45-8. [3]. Denis Fourches, et al. Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species. Chem Res Toxicol. 2010 Jan;23(1):171-83. [4]. Fu-Chi Kang, et al. Apoptotic effect of cordycepin combined with cisplatin and/or paclitaxel on MA-10 mouse Leydig tumor cells. Onco Targets Ther. 2015 Sep 1:8:2345-60. [5]. Fujun Zhou, et al. Translational control of Arabidopsis meristem stability and organogenesis by the eukaryotic translation factor eIF3h. PLoS One. 2014 Apr 15;9(4):e95396.

2'-Deoxyinosine, C10H12N4O4 CAS NO.890-38-0 MW252.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥96.0%, OR[α](C=1 g/100ml H2O): -23.6°, mp250°C, bp681.2°C at 760 mmHg, Flash point 365.8°C, Storage: Storage temp. -20°C, Applications 2’-deoxyadenosine has the activity of inhibiting the growth of human cancer cell lines and it was found to be associated with the lack of pur nucleotide phosphorylase. [1]. Bemi V, et al. Deoxyadenosine metabolism in a human colon-carcinoma cell line (LoVo) in relation to its cytotoxic effect in combination with deoxycoformycin. Int J Cancer. 1998 Mar 2;75(5):713-20. [2]. Charlotte Stentoft, et al. Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2014 Aug 22:1356:197-210. [3]. L S Bazar, et al. Mutation identification DNA analysis system (MIDAS) for detection of known mutations. Electrophoresis. 1999 Jun;20(6):1141-8. [4]. Le-Yue Du, et al. Comparative characterization of nucleotides, nucleosides and nucleobases in Abelmoschus manihot roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Dec 1:1006:130-137. [5]. Narender Pottabathini, et al. Synthesis and reactions of 2-chloro- and 2-tosyloxy-2'-deoxyinosine derivatives. J Org Chem. 2005 Sep 2;70(18):7188-95.

5-Methylcytidine, C10H15N3O5 CAS NO.2140-61-6 MW257.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp212-215°C, bp537.5°C at 760 mmHg, Storage: -20°C, protect from light, stored under nitrogen, [1]. Adolfo Lopez Torres, et al. Selective derivatization of cytosine and methylcytosine moieties with 2-bromoacetophenone for submicrogram DNA methylation analysis by reversed phase HPLC with spectrofluorimetric detection. Anal Chem. 2011 Oct 15;83(20):7999-8005. [2]. Arvindhan Nagarajan, et al. PEA15 regulates the DNA damage-induced cell cycle checkpoint and oncogene-directed transformation. Mol Cell Biol. 2014 Jun;34(12):2264-82. [3]. Azizollah Bakhtari, et al. The interfering effects of superovulation and vitrification upon some important epigenetic biomarkers in mouse blastocyst. Cryobiology. 2014 Dec;69(3):419-27. [4]. Caroline G Walker, et al. Modulation of the immune system during postpartum uterine inflammation. Physiol Genomics. 2015 Apr;47(4):89-101. [5]. Emilia Daghir-Wojtkowiak, et al. Least absolute shrinkage and selection operator and dimensionality reduction techniques in quantitative structure retention relationship modeling of retention in hydrophilic interaction liquid chromatography. J Chromatogr A. 2015 Jul 17:1403:54-62.

2′-O-Methyluridine, C10H14N2O6 CAS NO.2140-76-3 MW258.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=0.6025, H2O): 42.2°, mp154-156 °C, Storage: Storage temp. 2-8°C, 2'-O-methyluridine is a naturally occurring product found in the RNA of archaea, bacteria, and eukaryotes, including rRNA snRNA, snoRNA, and tRNA. [1]. Aučynaitė A, et al. Identification of a 2'-O-Methyluridine Nucleoside Hydrolase Using the Metagenomic Libraries. Molecules. 2018;23(11):2904. Published 2018 Nov 7.
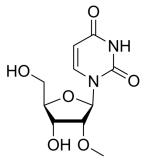
5-Methyluridine, C10H14N2O6 CAS NO.1463-10-1 MW258.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 95.61%, Water(KF): 0.26%, Residue on Ignition: 0.04%, Optical Rotation: -8.9°(C=1 g/100ml H2O), mp183-184°C, Storage: Storage temp. 2-8°C, [1]. Yang TH, et al. Intracellular levels of S-adenosylhomocysteine but not homocysteine are highly correlated to the expression of nm23-H1 and the level of 5-methyldeoxycytidine in human hepatoma cells with different invasion activities. Nutr Cancer. 2006;55(2):224-31. [2]. Aleksandra Krstulja, et al. Evaluation of molecularly imprinted polymers using 2',3',5'-tri-O-acyluridines as templates for pyrimidine nucleoside recognition. Anal Bioanal Chem. 2014 Oct;406(25):6275-84. [3]. Bruce S Ross, et al. Kilo-scale synthesis process for 2'-O-(2-methoxyethyl)-pyrimidine derivatives. Nucleosides Nucleotides Nucleic Acids. 2005;24(5-7):815-8. [4]. Emilia Daghir-Wojtkowiak, et al. Least absolute shrinkage and selection operator and dimensionality reduction techniques in quantitative structure retention relationship modeling of retention in hydrophilic interaction liquid chromatography. J Chromatogr A. 2015 Jul 17:1403:54-62. [5]. Juan Xiong, et al. Improved synthesis of 2'-deoxyadenosine and 5-methyluridine by Escherichia coli using an auto-induction system. World J Microbiol Biotechnol. 2012 Feb;28(2):721-7.
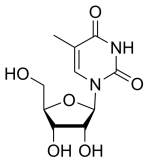
1-((2R,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, 1-beta-D-Arabinofuranosyluracil-2-C-methyl, 1-(2'-C-methyl-β-D-arabinofuranosyl)uracil, C10H14N2O6 CAS NO.114262-49-6 MW 258.23, mp132-135 °C
-3,4-dihydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
Clevudine, L-FMAU, C10H13FN2O5 CAS NO.163252-36-6 MW260.22, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.50%, mp184-185 °C, Storage: Storage temp. 2-8°C, Applications Clevudine (Levovir, L-FMAU) is an antiviral drug used to treat hepatitis B. [1]. Asselah T, et al. Clevudine: a promising therapy for the treatment of chronic hepatitis B. Expert Opin Investig Drugs. 2008;17(12):1963-1974. [2]. Smee DF, et, al. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother. 2008;19(3):115-24. [3]. Yoo BC, et al. Clevudine is highly efficacious in hepatitis B e antigen-negative chronic hepatitis B with durable off-therapy viral suppression. Hepatology. 2007;46(4):1041-1048.
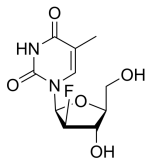
PSI-6206, RO 2433, GS-331007, 1-(3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, C10H13FN2O5 CAS NO.863329-66-2 MW260.22, form Solid, color White to off-white, Assay (HPLC): 98.11%, mp237-238 °C, Storage: Storage temp. 2-8°C, PSI-6206 (RO2433) is a selective HCV RNA polymerase inhibitor. [1]. Clark JL, et al. Design, Synthesis, and Antiviral Activity of 2'-Deoxy-2'-fluoro-2'-C-methyl-cytidine, a Potent Inhibitor of Hepatitis C Virus Replication. J Med Chem. 2005 Aug 25;48(17):5504-8. [2]. Ma H, et al. Characterization of the metabolic activation of hepatitis C virus nucleoside inhibitor beta-D-2'-Deoxy-2'-fluoro-2'-C-methylcytidine (PSI-6130) and identification of a novel active 5'-triphosphate species. J Biol Chem. 2007 Oct 12;282(41):29 [3]. Wang P, et al. An efficient and diastereoselective synthesis of PSI-6130: a clinically efficacious inhibitor of HCV NS5B polymerase. J Org Chem. 2009 Sep 4;74(17):6819-24.

1-((2S,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, 1-(2-Deoxy-2-fluoro-2-methyl-α-D-ribofuranosyl)-2,4(1H,3H)-pyrimidinedione, C10H13FN2O5 CAS NO.2041584-99-8 MW260.22, form Solid, color Light yellow to brown, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 96.75%, Storage: Storage temp. 2-8°C
-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
1-((2S,3S,4S,5S)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, C10H13FN2O5 CAS NO.1946820-96-7 MW260.22, Assay 97%, Storage: Store at room temperature
-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
4-Thiouridine, C9H12N2O5S CAS NO.13957-31-8 MW260.27, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, mp139-140 °C, Storage: Storage temp. -20°C, 4-Sulfuridine plays a role in evaluating the synthesis of new RNA. It also enhances site-specific crosslinking. 4-Suridine can also be used as a photoaffinity probe due to its stability, even in the absence of specific photoactivation. It can also be integrated into RNA. [1]. Burger K, et al. 4-thiouridine inhibits rRNA synthesis and causes a nucleolar stress response. RNA Biol. 2013 Oct;10(10):1623-30. [2]. Anne Seifert, et al. Proteotoxic stress reprograms the chromatin landscape of SUMO modification. Sci Signal. 2015 Jul 7;8(384):rs7. [3]. David J Duffy, et al. Integrative omics reveals MYCN as a global suppressor of cellular signalling and enables network-based therapeutic target discovery in neuroblastoma. Oncotarget. 2015 Dec 22;6(41):43182-201. [4]. Jungwook Kim, et al. Structural basis for hypermodification of the wobble uridine in tRNA by bifunctional enzyme MnmC. BMC Struct Biol. 2013 Apr 24:13:5. [5]. K J Sokoloski, et al. Noncapped Alphavirus Genomic RNAs and Their Role during Infection. J Virol. 2015 Jun;89(11):6080-92.

5-Fluorocytidine, C9H12FN3O5 CAS NO.2341-22-2 MW261.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp196-200°C, bp511.4±60.0 °C at 760 mmHg, Storage: 2-8°C, stored under nitrogen, 5-Fluorocytidine has antiviral activity. [1]. Grosso LE, et al. Alterations in the maturation and structure of ribosomal precursor RNA in Novikoff hepatoma cells induced by 5-fluorocytidine. Biochemistry. 1984 Jun 5;23(12):2651-6.
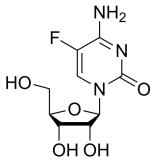
2-Thio-6-azauridine, C8H11N3O5S CAS NO.27089-56-1 MW261.26, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR[α](C=0.50g/100ml H2O): -80.5°, mp201-203 °C, Storage: 2-8°C, stored under nitrogen

5-Fluorouridine, 1-(3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-fluoropyrimidine-2,4(1H,3H)-dione, C9H11FN2O6 CAS NO.316-46-1 MW262.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structureMS: Consistent with structure, Assay (HPLC): 98.01%, mp182-184°C, Storage: 2-8°C, stored under nitrogen, 5-Fluorouridine (FUrd) is cytotoxic to cancer cells. FUrd is often used in chemical and biochemical studies with fluorouracil and thymidine analogs. [1]. Houghton JA, et, al. Mechanism of induction of gastrointestinal toxicity in the mouse by 5-fluorouracil, 5-fluorouridine, and 5-fluoro-2'-deoxyuridine. Cancer Res. 1979 Jul;39(7 Pt 1):2406-13. [2]. Schmittgen TD, et, al. Diverse gene expression pattern during 5-fluorouridine-induced apoptosis. Int J Oncol. 2005 Aug;27(2):297-306. [3]. Wu FL, et, al. Gelatinases-stimuli nanoparticles encapsulating 5-fluorouridine and 5-aza-2'-deoxycytidine enhance the sensitivity of gastric cancer cells to chemical therapeutics. Cancer Lett. 2015 Jul 10;363(1):7-16. [4]. Edward J Miracco, et al. The products of 5-fluorouridine by the action of the pseudouridine synthase TruB disfavor one mechanism and suggest another. J Am Chem Soc. 2011 Aug 10;133(31):11826-9. [5]. Ellen J B Derissen, et al. Development of an LC-MS/MS assay for the quantitative determination of the intracellular 5-fluorouracil nucleotides responsible for the anticancer effect of 5-fluorouracil. J Pharm Biomed Anal. 2015 Jun 10:110:58-66.
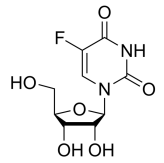
Gemcitabine, 4-amino-1-(3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one, C9H11F2N3O4 CAS NO.95058-81-4 MW263.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structureLCMS: Consistent with structure, Assay (LCMS): 99.39%, mp168.64°C, bp482.7°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Gagnadoux F, et al. Safety of pulmonary administration of gemcitabine in rats. J Aerosol Med. 2005 Summer;18(2):198-206 [2]. Wang H, et al. Enhanced efficacy of Gemcitabine by indole-3-carbinol in pancreatic cell lines: the role of human equilibrativenucleoside transporter 1. Anticancer Res. 2011 Oct;31(10):3171-80. [3]. Yip-Schneider MT, et al. Dimethylaminoparthenolide and Gemcitabine: a survival study using a genetically engineered mouse model of pancreatic cancer. BMC Cancer. 2013 Apr 17;13:194. [4]. Yusheng Cai, et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020 Jul;30(7):574-589.

7-Deaza-2'-dG, 7-Deaza-2'-deoxyguanosine, 7-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-imino-1H-pyrrolo[2,3-d]pyrimidin-4-ol, C11H14N4O4 CAS NO.86392-75-8 MW266.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 97.45%, mp262-265 °C, Storage: 2-8°C, protect from light

Vidarabine, Ara-A, Adenine Arabinoside, 9-β-D-Arabinofuranosyladenine, Adenine 9-β-D-arabinofuranoside, C10H13N5O4 CAS NO.5536-17-4 MW267.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.36%, OR[α](C=1.03 g/100ml DMF): -3.0°, mp260°C, bp410.43 °C at 760 mmHg, Storage: Storage temp. 2-8°C, Cell-permeable adenosine triphosphatase inhibitor; IC50 = 30 μM in rat brain preparation dispersed by deterg. Clinically significant antiviral agent, especially against herpes simplex (HSV), by inhibition of DNA polymerase. [1]. Donald F Smee, et al. A review of compounds exhibiting anti-orthopoxvirus activity in animal models. Antiviral Res. 2003 Jan;57(1-2):41-52. [2]. Suzuki M, et al. Synergistic antiviral activity of acyclovir and vidarabine against herpes simplex virus types 1 and 2 and varicella-zoster virus. Antiviral Res. 2006;72(2):157-161. [3]. Whitley, R., et al., Vidarabine: a preliminary review of its pharmacological properties and therapeutic use. Drugs, 1980. 20(4): p. 267-82. [4]. Aneel Paulus, et al. The investigational agent MLN2238 induces apoptosis and is cytotoxic to CLL cells in vitro, as a single agent and in combination with other drugs. Br J Haematol. 2014 Apr;165(1):78-88. [5]. Denis Fourches, et al. Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species. Chem Res Toxicol. 2010 Jan;23(1):171-83.
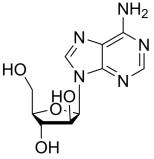
Adenosine, Adenine riboside, D-Adenosine, C10H13N5O4 CAS NO.58-61-7 MW267.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.06%, mp234-236°C, bp676.3°C at 760 mmHg , Storage: Storage temp. 2-8°C, Adenosine is active in cardiovascular diseases. Indicated in angina pectoris due to its property of increasing coronary artery output and in hypertension due to vasodilatory effect. It is also active in peripheral circulation, dilating the capillaries. It is also recommended for the treatment of granulocytopenia It induces apoptosis in human leukemia HL-60 cells and also has antiarrhythmic properties. [1]. Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018;98(3):1591-1625. [2]. Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111(4):904-915. [3]. Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315-1323. [4]. Zhou XT, et al. Inhibition of autophagy enhances adenosine induced apoptosis in human hepatoblastoma HepG2 cells. Oncol Rep. 2019;41(2):829-838. [5]. Ashleigh M Byrne, et al. The activity of cAMP-phosphodiesterase 4D7 (PDE4D7) is regulated by protein kinase A-dependent phosphorylation within its unique N-terminus. FEBS Lett. 2015 Mar 12;589(6):750-5.

2'-Deoxyguanosine, Deoxyguanosine, Guanine deoxyriboside, C10H13N5O4 CAS NO.961-07-9 MW267.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.75%, mp300 °C, bp725.475 °C at 760 mmHg, Storage: Storage temp. 2-8°C, 2'-Deoxyguanosine (dG) is a purine nucleoside that, after successive phosphorylation (kinase), forms dG, which is used by DNA polymerases and reverse transcriptases to synthesize DNA. Deoxyguanosine is the most electron-rich of the four canonical bases, including nucleophilic sites that are susceptible to oxidative damage. This makes deoxyguanosine and its oxidized derivatives useful reagents for studying the mechanisms of oxidative to nucleosides and nucleotides. [1]. Deoxyguanosine, From Wikipedia

Inosine, C10H12N4O5 CAS NO.58-63-9 MW268.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, mp222-226°C, bp226 °C at 760 mmHg, Storage: Storage temp. 2-8°C, Inosine is an effective stimulator of nerve growth factor (NGF) induced nerve sprouting. The increase in adenosine level in the brain after is associated with increased expression of proteins related to axonal regeneration and growth. Mice treated with adenosine showed enhanced recovery of fine motor control after ischemic brain injury.enosine can be used to study the A-to-I RNA editing process. [1]. Ajith A. Welihinda, et al. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal. 2016 Jun; 28(6): 552-560. [2]. Filipe Marques Gonçalves, et al. Signaling pathways underlying the antidepressant-like effect of inosine in mice. Purinergic Signal. 2017 Jun; 13(2): 203-214. [3]. Francisney Pinto Nascimento, et al. Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological, genetic and biochemical aspects. Mol Neurobiol. 2015;51(3):1368-78. [4]. Sara Cipriani, et al. Protection by inosine in a cellular model of Parkinson’s disease. Neuroscience. 2014 Aug 22; 274: 242-249. [5]. Arun Sreekumar, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4.
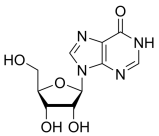
2′-Deoxy-2′-fluoroadenosine, C10H12FN5O3 CAS NO.64183-27-3 MW269.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp228-231 °C, bp628.6±65.0 °C at 760 mmHg, Storage: 2-8°C, stored under nitrogen, 2'-Fluoro-2'-deoxyadenosine is a potential pro-drug of 2-fluroadenine, involving deoxyation through the reduction of the 2'-thiocarbonyl imidazolinium with (Tms)3SiH as the hydrogen donor. [1]. A E Hassan, et al. A convenient synthesis of 2'-deoxy-2-fluoroadenosine; a potential prodrug for suicide gene therapy. Nucleosides Nucleotides Nucleic Acids. 2000 Mar;19(3):559-65.
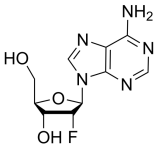
2'-Deoxyadenosine monohydrate, C10H15N5O4 CAS NO.16373-93-6 MW269.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp185-187°C, bp627.2°C at 760 mmHg, Storage: 2-8°C, protect from light, 2'-Deoxyadenosine (2'-dAdo) is a deoxyribose nucleoside that can be used by certain cells as an energy under energy stress conditions and affects cAMP levels. 2'-dAdo is used in the study of the function of adenosine analogs on various biological processes, [1]. I Remy, et al. Detection and visualization of protein interactions with protein fragment complementation assays. Methods Mol Biol. 2002:185:447-59. [2]. M Nakamura, et al. Regulation of glucose metabolism by adenine nucleotides in round spermatids from rat testes. J Biol Chem. 1982 Dec 10;257(23):13945-50. [3]. Martha J Folmsbee, et al. Anaerobic growth of Bacillus mojavensis and Bacillus subtilis requires deoxyribonucleosides or DNA. Appl Environ Microbiol. 2004 Sep;70(9):5252-7. [4]. O Magnus, et al. Provocation testing of human sperm motility using energy substrates and activators of the cyclic nucleotide system: II. Studies on sperm from asthenozoospermic subjects. Int J Fertil. 1993 Mar-Apr;38(2):123-8. [5]. Sonya M Schuh, et al. Adenosine and catecholamine agonists speed the flagellar beat of mammalian sperm by a non-receptor-mediated mechanism. Biol Reprod. 2007 Dec;77(6):960-9.

(2R,3S,5R)-5-(6-Chloro-9H-purin-9-yl)-2-(hydroxymethyl)tetrahydrofuran-3-ol, C10H11ClN4O3 CAS NO.4594-45-0 MW270.67, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.42%, Optical Rotation: -12.0°(C=1g/100ml MEOH), bp568°C at 760 mmHg, Flash point 297.3°C, Storage: -20°C, sealed storage, away from moisture, 6-Chloropurine 2'-deoxyriboside is used for the synthesis of 9-(2,3-dideoxy-2-flu-beta-D-threo-pentofuranosyl)adenine.
-5-(6-Chloro-9H-purin-9-yl)-2-(hydroxymethyl)tetrahydrofuran-3-ol.png)
Etheno-2'-deoxy-Beta-D-adenosine, 3-(2-Deoxy-β-D-erythro-pentofuranosyl)-3H-imidazo[2,1-i]purine, 1,N6-Etheno-2'-deoxyadenosine, 1,N6-Etheno-dA, 1,N6-Ethenodeoxyadenosine;Ethenodeoxyadenosine, N1,N6-Etheno-2'-deoxyadenosine, C12H13N5O3 CAS NO.68498-25-9 MW275.26, form Solid, color White to Light Beige Pale Beige to Light Beige, NMR Conforms to Structure Conforms, Elemental Analysis Conforms %C: 48.79, %H: 5.17, %N: 23.54 HPLC Assay Report Result 98.70% (230 nm), MS Conforms to Structure Conforms, Storage: -20°C, An etheno DNA-adduct of adenosine found in atherosclerotic lesions in aorta smooth muscle cells induced via lipid peroxidation. A biomarker for genotoxicity. Nair, J. et al.: Mut. Res. Fund. Mol. Mech. Mutagen., 621, 95 (2007); Meerang, M. et al.: Free Rad. Biol. Med., 44, 1863 (2008); Brink, A. et al.: Mut. Res. Gen. Toxicol. Env. Mutagen., 678, 123 (2009)

1-((2R,3R,4R,5R)-3-chloro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, C10H13ClN2O5 CAS NO.1496551-72-4 MW276.68, Assay 98%, Storage: 2-8 °C protect from light, Chloro Sofosbuvir Desphosphate is a possible metabolite of Sofosbuvir (P839640) which is a prodrug that is metabolized to the active antiviral agent 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-monophosphate and is currently being investigated in phase 3 clinical trials for the treatment of hepatitis C. [1]Lam, A.M.,et al.: Antimicrob. Agents. Chemotherapy., 56, 3359 (2012); Lam, A.M., et al.: J. Virol., 85, 12334 (2011); Sofia, M.J., et al.: J. Medn. Chem., 53, 7202 (2010)
-3-chloro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
Cytarabine (hydrochloride), Cytosine β-D-arabinofuranoside hydrochloride, Cytosine Arabinoside hydrochloride, Ara-C hydrochloride, C9H14ClN3O5 CAS NO.69-74-9 MW279.68, form Solid, color White to off-white, Assay: ≥95.0%, mp197-198°C, bp545.7°C at 760 mmHg, Flash point 283.8°C, Storage: 2-8°C, sealed storage, away from moisture, Ara-C is phosphorylated to Ara-CTP and incorporated into DNA. It inhibits DNA replication by forming a cleaving complex with topoisase I, resulting in DNA fragmentation and ultimately inducing apoptosis through the PKC signaling pathway. Does not inhibit RNA synthesis. Antileukemic agent. [1]. Besirli, C.G., et al. Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ, 2003. 10(9): p. 1045-58. [2]. Renis HE. Antiviral activity of cytarabine in herpesvirus-infected rats. Antimicrob Agents Chemother. 1973 Oct;4(4):439-44. [3]. Richel, D.J., et al., Comparison of the antileukaemic activity of 5 aza-2-deoxycytidine and arabinofuranosyl-cytosine in rats with myelocytic leukaemia. Br J Cancer, 1988. 58(6): p. 730-3. [4]. Shepshelovich D, et al. Pharmacodynamics of cytarabine induced leucopenia: a retrospective cohort study. Br J Clin Pharmacol. 2015 Apr;79(4):685-91. [5]. Tobias, S.C. and R.F. Borch, Synthesis and biological evaluation of a cytarabine phosphoramidate prodrug. Mol Pharm, 2004. 1(2): p. 112-6.
.png)
N6-Methyladenosine, 6-Methyladenosine, N-Methyladenosine, C11H15N5O4 CAS NO.1867-73-8 MW281.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=0.6 g/100ml H2O): -59.3°, mp172 °C, bp649.1°C at 760 mmHg, Storage: Storage temp. 2-8°C, N6-methyladenosine is the most prevalent internal (non-cap) modification in messenger RNA (mRNA) of all higher eukaryotes, [1]. Dang W, et al. N6-Methyladenosine and Viral Infection. Front Microbiol. 2019 Mar 5;10:417. [2]. Li Y, et al. Genome-wide detection of high abundance N6-methyladenosine sites by microarray. RNA. 2015 Aug;21(8):1511-8. [3]. Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014 Jan 2;505(7481):117-20.

2'-O-Methyladenosine, C11H15N5O4 CAS NO.2140-79-6 MW281.272, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.94%, Optical Rotation[a]: -50.5°(C=0.01 g/mL H2O), mp200-202°C, bp623.8°C at 760 mmHg, Flash point 331°C, Storage: -20°C, protect from light, 2'-O-Methyladenosine is an adenosine analog used in the preparation of nucleoside derivatives as inhibitors of RNA-dependent RNA virusase. [1]. Hirschhorn R, et al. Increased excretion of modified adenine nucleosides by children with adenosine deaminase deficiency. Pediatr Res. 1982 May;16(5):362-9. [2]. Yamada T, et al. Naturally occurring 2'-O-methylpurine nucleosides with hypotensive properties. Cell Mol Life Sci. 1998 Feb;54(2):125-8. [3]. A Theibert, et al. Adenosine and its derivatives inhibit the cAMP signaling response in Dictyostelium discoideum. Dev Biol. 1984 Nov;106(1):166-73. [4]. C G Edmonds, et al. Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria). J Bacteriol. 1991 May;173(10):3138-48. [5]. E N Kalinichenko, et al. [Substrate specificity of adenosine deaminase. The role of methyl groups at 2', 3', and 5'-carbon atoms of adenosine]. Bioorg Khim. 1988 Sep;14(9):1157-61.
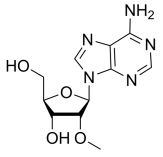
8-Hydroxy-2'-deoxyguanosine, 8-Oxo-2-Deoxyguanosine, CAS NO.88847-89-6 MW283.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 95.38%, mp217-220 °C, Storage: Storage temp. 2-8°C, A biomarker compound, typically indicative of DNA damage associated with mutagenesis and carcinogenicity. The level of 8-hydroxy-2'-deguanosine helps to assess oxidative stress and DNA damage in individuals afflicted with laryngeal cancer. Therefore, it is a major biomarker of DNA damage induced reactive oxygen species (ROS). [1]. Valavanidis A, et al. 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009 Apr;27(2):120-39. [2]. Francisco Ortiz, et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J Pineal Res. 2015 Jan;58(1):34-49. [3]. Fumiyo Takabayashi, et al. Viscous methyl cellulose solution thickens gastric mucosa and increases the number of gland mucous cells in mice. Br J Nutr. 2013 Oct;110(7):1195-200. [4]. Gwan Ui Hong, et al. Expression of airway remodeling proteins in mast cell activated by TGF-β released in OVA-induced allergic responses and their inhibition by low-dose irradiation or 8-oxo-dG. Radiat Res. 2014 Apr;181(4):425-38. [5]. Li Mu, et al. L-cysteine: a biocompatible, breathable and beneficial coating for graphene oxide. Biomaterials. 2015 Jun:52:301-11.

Guanosine, DL-Guanosine, Vernine, 2-(2-amino-6-hydroxy-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol, C10H13N5O5 CAS NO.118-00-3 MW283.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 99.02%, mp250°C, bp425.8 °C at 760 mmHg, Storage: 2-8°C protect from light, Guanosine as a guanine-based purine system induces cellular effects. It regulates the uptake of glutamate by glutate transporter. It may have a neuroprotective function in central nervous system diseases. Guanylic acid promotes axonal arborization, growth, proliferation, and differentiation. administration of guanylic acid supplemented GTP and induced a protective function in renal ischemic injury. [1]. De Clercq E1. Guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids. 2000 Oct-Dec;19(10-12):1531-41. [2]. Carina Villacrés, et al. Low glucose depletes glycan precursors, reduces site occupancy and galactosylation of a monoclonal antibody in CHO cell culture. Biotechnol J. 2015 Jul;10(7):1051-66. [3]. Christian D Laourdakis, et al. Comprehensive quantitative analysis of purines and pyrimidines in the human malaria parasite using ion-pairing ultra-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Sep 15:967:127-33. [4]. Chunjing Feng, et al. Hairpin assembly circuit-based fluorescence cooperative amplification strategy for enzyme-free and label-free detection of small molecule. Talanta. 2015 Oct 1:143:101-106. [5]. Dominique Thomas, et al. Quantitation of endogenous nucleoside triphosphates and nucleosides in human cells by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2015 May;407(13):3693-704.
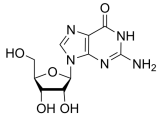
Xanthosine, 9-(3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purine-2,6-diol, C10H12N4O6 CAS NO.146-80-5 MW284.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp174-176 °C, Storage: Storage temp. -20°C, Xanthosine is a nucleoside derived from Xanthine and ribose. [1]. Choudhary S, et, al. Examination of the xanthosine response on gene expression of mammary epithelial cells using RNA-seq technology. J Anim Sci Technol. 2018 Jul 13;60:18.
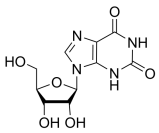
6-Thioinosine, 6-Mercaptopurine riboside, 2-(hydroxymethyl)-5-(6-mercapto-9H-purin-9-yl)tetrahydrofuran-3,4-diol, C10H12N4O4S CAS NO.574-25-4 MW284.297, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, mp220-223 °C, bp714.7±70.0 °C at 760 mmHg, Storage: -20°C, stored under nitrogen, [1]. Lee J, et al. Anti-adipogenesis by 6-thioinosine is mediated by downregulation of PPAR gamma through JNK-dependent upregulation of iNOS. Cell Mol Life Sci. 2010 Feb;67(3):467-81. [2]. Neubert D, et al. Interference of 6-mercaptopurine riboside, 6-methylmercaptopurine riboside and azathioprine with the morphogenetic differentiation of mouse extremities in vivo and in organ culture. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jun;298(2):93-105.

4'-C-Azidouridine, 4'-Azidouridine, C9H11N5O6 CAS NO.139442-01-6 MW285.21, Assay 98%, [1]. Connolly GP, et al. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci. 1999 May;20(5):218-25.
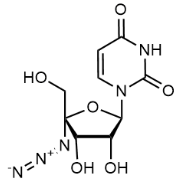
N4-Acetylcytidine, C11H15N3O6 CAS NO.3768-18-1 MW285.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.66%, Water(KF): 0.03%, Optical Rotation: 39.50°(C=0.01g/mL, H2O, 20°C, 589nm), mp199°C, Storage: Storage temp. -20°C, [1]. Clement T Y Chan, et al. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010 Dec 16;6(12):e1001247. [2]. Emilia Daghir-Wojtkowiak, et al. Least absolute shrinkage and selection operator and dimensionality reduction techniques in quantitative structure retention relationship modeling of retention in hydrophilic interaction liquid chromatography. J Chromatogr A. 2015 Jul 17:1403:54-62. [3]. G S Morris, et al. Use of biological fluids for the rapid diagnosis of potentially lethal inherited disorders of human purine and pyrimidine metabolism. Biomed Chromatogr. 1986 Jun;1(3):109-18. [4]. J G Tebib, et al. Relationship between urinary excretion of modified nucleosides and rheumatoid arthritis process. Br J Rheumatol. 1997 Sep;36(9):990-5. [5]. Lucas Willmann, et al. Exometabolom analysis of breast cancer cell lines: Metabolic signature. Sci Rep. 2015 Aug 21:5:13374.

6-Chloro-7-deazapurine-β-D-riboside, C11H12ClN3O4 CAS NO.16754-80-6 MW285.68, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: 2-8°C, stored under nitrogen, [1]. Hajime Iwamura, et al. Antifungal Activity of Substituted 7-(β-D-Ribofuranosyl)pyrrolo-[2,3-d]pyrimidines. Agricultural and Biological Chemistry, Volume 40, Issue 7, 1 July 1976, Pages
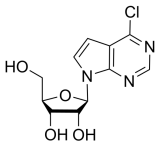
5'-Chloro-5'-deoxyadenosine, C10H12ClN5O3 CAS NO.892-48-8 MW285.69, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥96.0%, mp187 °C, bp645.6±65.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C

Cladribine, 2-Chloro-2′-deoxyadenosine, C10H12ClN5O3 CAS NO.4291-63-8 MW285.69, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, mp181-185°C, bp387.1°C at 760 mmHg, Storage: 2-8°C, protect from light, Deoxyadenosine analogues resistant to adenosine deaminase; anti-leukemic with immunosuppressive activity

Entecavir (monohydrate), C12H17N5O4 CAS NO.209216-23-9 MW295.29, form Solid, color White to off-white, LCMS: Consistent with structure, Assay (LCMS): 98.58%, mp>220°C, bp734.2°C at 760 mmHg, Storage: Storage temp. 2-8°C
.png)
Trifluridine, Trifluorothymidine, 5-Trifluorothymidine, C10H11F3N2O5 CAS NO.70-00-8 MW296.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.85%, mp190-193°C, Storage: Storage temp. 2-8°C, Trifluridine is a thymidine analog that is photosensitive. TFT is used as a substrate for thymidine kinase study enzyme specificity and kinetics. The phosphorylation of TFT into DNA induces damage, making it useful for DNA repair studies. TFT can also be used to thymidylate synthase and screen for mutated thymidine kinase genes. It induces antitumor activity in gastrointestinal (GI) cancers and has potential for herpetic keratitis. [1]. Jia HJ, et al. Trifluridine induces HUVECs senescence by inhibiting mTOR-dependent autophagy. Biochem Biophys Res Commun. 2022 Jun 25;610:119-126. [2]. Li J, et al. Trifluridine selectively inhibits cell growth and induces cell apoptosis of triple-negative breast cancer. Am J Cancer Res. 2020 Feb 1;10(2):507-522. [3]. Okayama T, et al. Involvement of concentrative nucleoside transporter 1 in intestinal absorption of trifluorothymidine, a novel antitumor nucleoside, in rats. J Pharmacol Exp Ther. 2012 Feb;340(2):457-62. [4]. Suzuki N,et al. Trifluridine/tipiracil increases survival rates in peritoneal dissemination mouse models of human colorectal and gastric cancer. Oncol Lett. 2017 Jul;14(1):639-646. [5]. Caitlin Lynch, et al. Quantitative high-throughput identification of drugs as modulators of human constitutive androstane receptor. Sci Rep. 2015 May 20:5:10405.

5'-Methylthioadenosine, 5'-(Methylthio)-5'-deoxyadenosine, 5'-Deoxy-5'-(methylthio)adenosine, 5'-S-Methyl-5'-thioadenosine, C11H15N5O3S CAS NO.2457-80-9 MW297.33, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp210-213 °C, bp642.7±65.0 °C at 760 mmHg, Storage: -20°C, protect from light, 5'-Deoxy-5'-(methylthio)adenosine (Methylthioadenosine) can be used as a substrate to the specificity and kinetics of 5'-methylthioadenosinephosphorylase (MTAP) (EC2.4.2.28, an enzyme that supports the expression of tumor suppressor genes and the S-adenosylmethionine (AdoMet) and methionine salvage pathways. [1]. Li Y, et al. 5'-Methylthioadenosine and Cancer: old molecules, new understanding. J Cancer. 2019;10(4):927-936. [2]. Tang B, et al. Specific Targeting of MTAP-Deleted Tumors with a Combination of 2'-Fluoroadenine and 5'-Methylthioadenosine. Cancer Res. 2018;78(15):4386-4395. [3]. Tang Y, et al. 5'-Methylthioadenosine attenuates ischemia reperfusion injury after liver transplantation in rats. Inflammation. 2014;37(5):1366-1373. [4]. Yaofeng Li, et al. 5'-Methylthioadenosine and Cancer: old molecules, new understanding. J Cancer. 2019;10(4):927-936. [5]. Ignacio Hernandez-Morato, et al. Differential expression of glial-derived neurotrophic factor in rat laryngeal muscles during reinnervation. Laryngoscope. 2014 Dec;124(12):2750-6.
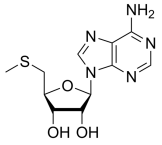
6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3,9-dihydro-2H-purine-2-thione, 2-Thioadenosine, C10H13N5O4S CAS NO.43157-50-2 MW299.31, form Solid, color Light yellow to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 96.73%, mp>178 °C, bp544.5±60.0 °C at 760 mmHg, Storage: 2-8°C, protect from light,
-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3,9-dihydro-2H-purine-2-thione.png)
Gemcitabine (hydrochloride), C9H12ClF2N3O4 CAS NO.122111-03-9 MW299.66, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp>250 °C, Storage: 2-8°C, sealed storage, away from moisture and light, For the treatment of inoperable advanced or metastatic pancreatic cancer and for the treatment of locally advanced or metastatic non-small cell lung cancer, of middle and late stage non-small cell lung cancer, non-small cell lung cancer, pancreatic cancer, bladder cancer, breast cancer and other solid tumors. [1]. Gagnadoux F, et al. Safety of pulmonary administration of gemcitabine in rats. J Aerosol Med. 2005 Summer;18(2):198-206 [2]. Lou M, et al. Physical interaction between human ribonucleotide reductase large subunit and thioredoxin increases colorectal cancer malignancy. J Biol Chem. 2017 Jun 2;292(22):9136-9149. [3]. Wang H, et al. Enhanced efficacy of Gemcitabine by indole-3-carbinol in pancreatic cell lines: the role of human equilibrativenucleoside transporter 1. Anticancer Res. 2011 Oct;31(10):3171-80 [4]. Yip-Schneider MT, et al. Dimethylaminoparthenolide and Gemcitabine: a survival study using a genetically engineered mouse model of pancreatic cancer. BMC Cancer. 2013 Apr 17;13:194. [5]. Andrew W Truman, et al. Quantitative proteomics of the yeast Hsp70/Hsp90 interactomes during DNA damage reveal chaperone-dependent regulation of ribonucleotide reductase. J Proteomics. 2015 Jan 1:112:285-300.
.png)
2-Chloroadenosine, C10H12ClN5O4 CAS NO.146-77-0 MW301.69, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.01% Optical Rotation: -53.1°(C=0.01g/ml DMSO), mp162°C, bp591.8±60.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Evans MC, et al. An adenosine analogue, 2-chloroadenosine, protects against long term development of ischaemic cell loss in the rat hippocampus.Neurosci Lett. 1987 Dec 29;83(3):287-92. [2]. Jarvis SM,2-Chloroadenosine, a permeant for the nucleoside transporter.Biochem Pharmacol. 1985 Sep 15;34(18):3237-41. [3]. Catherine M Davis, et al. Ultrasound stimulates formation and release of vasoactive compounds in brain endothelial cells. Am J Physiol Heart Circ Physiol. 2015 Aug 15;309(4):H583-91. [4]. D B J Bone, et al. Hypoxanthine uptake by skeletal muscle microvascular endothelial cells from equilibrative nucleoside transporter 1 (ENT1)-null mice: effect of oxidative stress. Microvasc Res. 2015 Mar:98:16-22. [5]. Derek B J Bone, et al. Oxidative stress modulates nucleobase transport in microvascular endothelial cells. Microvasc Res. 2014 Sep:95:68-75.
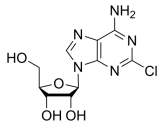
2'-O-(2-Methoxyethyl)-uridine,C12H18N2O7 CAS NO.223777-15-9 MW302.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: 2-8°C, protect from light, [1]. UrtziLegorburu, et al. Conversion of uridine into 2′-O-(2-methoxyethyl)uridine and 2′-O-(2-methoxyethyl)cytidine. Tetrahedron
-uridine.png)
5-BrdU, 5-Bromo-2'-deoxyuridine, C9H11BrN2O5 CAS NO.59-14-3 MW307.10, form Solid, color White to off-white, Assay: ≥95.0%, mp191-194°C, Storage: 2-8°C, protect from light, 5-Bromo-2'-deoxyuridine (5-BrdU) is a thymidine analog that is incorporated into DNA. 5-BrdU is routinely and widely used to measure DNA synthesis and label dividing cells. Thus, 5-BrdU is used to study cellular signaling and other processes induce cell proliferation. [1]. Levkoff LH, et al. Bromodeoxyuridine inhibits cancer cell proliferation in vitro and in vivo. Neoplasia. 2008 Aug;10(8):804-16. [2]. Rothaeusler K, et al. Assessment of cell proliferation by 5-bromodeoxyuridine (BrdU) labeling for multicolor flow cytometry. Curr Protoc Cytom. 2007 Apr;Chapter 7:Unit7.31 [3]. Ahmed M Osman, et al. Transplantation of enteric neural stem/progenitor cells into the irradiated young mouse hippocampus. Cell Transplant. 2014;23(12):1657-71. [4]. Cheng-Hsiang Kuo, et al. FGFR1 mediates recombinant thrombomodulin domain-induced angiogenesis. Cardiovasc Res. 2015 Jan 1;105(1):107-17. [5]. Denise Zwanziger, et al. The impact of CLAUDIN-1 on follicular thyroid carcinoma aggressiveness. Endocr Relat Cancer. 2015 Oct;22(5):819-30.

2',3'-O-Isopropylideneadenosine, C13H17N5O4 CAS NO.362-75-4 MW307.31, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.87%, Water(KF): 0.33%, OR(C=1.00 g/100ml, 1,4-dioxane): -104.5°, mp221-222°C, bp447.81 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Man S, et al. Potential and promising anticancer drugs from adenosine and its analogs. Drug Discov Today. 2021 Jun;26(6):1490-1500.
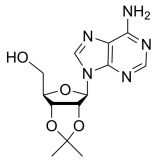
9-((3aR,4R,6R,6aR)-6-(Hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-ol, 9-[2,3-O-(1-methylethylidene)pentofuranosyl]-3,9-dihydro-6H-purin-6-one, C13H16N4O5 CAS NO.2140-11-6 MW308.29, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=0.99 g/100ml MEOH): -62.0°, mp263-272°C, bp606.6°C at 760 mmHg, Flash point 320.7°C, Storage: Storage temp. 2-8°C
-6-(Hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-ol.png)
N6-(2-Hydroxyethyl)adenosine, C12H17N5O5 CAS NO.4338-48-1 MW311.29, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp194-195°C, bp725.8°C at 760 mmHg, Storage: 2-8°C, protect from light, It is an anticonvulsant that works by activating the adenosine A1 receptor (AA1R). [1]. Man S, et al. Potential and promising anticancer drugs from adenosine and its analogs. Drug Discov Today. 2021 Jun;26(6):1490-1500. [2]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.
adenosine.png)
2'-O-MOE-5-Me-rU, 2'-O-(2-Methoxyethyl)-5-methyl-uridine, C13H20N2O7 CAS NO.163759-49-7 MW316.31, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 98.09%, mp115.5-116.5 °C, Storage: 2-8°C, protect from light

2,6-Dichloropurine riboside, (2R,3R,4S,5R)-2-(2,6-Dichloro-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol, 2,6-Dichloropurine-9-Beta-D-riboside, C10H10Cl2N4O4 CAS NO.13276-52-3 MW321.12, form Solid, color White to off-white 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.97%, OR(C=1.00 g/100ml, H2O ): -30.1°, mp>, 190°C, bp589.3°C at 760 mmHg, Storage: -20°C, sealed storage, away from moisture

5-Bromocytidine, C9H12BrN3O5 CAS NO.3066-86-2 MW322.11, form SOlid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 97.96%, mp183-185 °C, bp555.4°C at 760 mmHg, Storage: Storage temp. 2-8°C
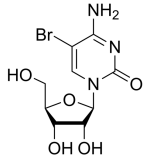
Cytidine 5'-monophosphate, 5'-Cytidylic acid, 5'-CMP, C9H14N3O8P CAS NO.63-37-6 MW323.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.99%, mp222°C, bp678°C at 760 mmHg, Storage: 2-8°C, protect from light, Nucleic acid components, separated from yeast nucleic acid. Used as food additives, genetic engineering reagents and pharmaceutical raw materials. [1]. Hernández AG, et, al. The determination of acid-soluble nucleotides in milk by improved enzymic methods: a comparison with the ion-exchange column chromatography procedure. J Sci Food Agric. 1981 Nov;32(11):1123-31. [2]. A Mukhopadhya, et al. The anti-inflammatory potential of a moderately hydrolysed casein and its 5 kDa fraction in in vitro and ex vivo models of the gastrointestinal tract. Food Funct. 2015 Feb;6(2):612-21. [3]. Amit Choudhary, et al. 4-ketoproline: An electrophilic proline analog for bioconjugation. Biopolymers. 2015 Mar;104(2):110-5. [4]. Arun Sreekumar, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [5]. Christian D Laourdakis, et al. Comprehensive quantitative analysis of purines and pyrimidines in the human malaria parasite using ion-pairing ultra-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Sep 15:967:127-33.

2′,3′-Isopropylideneguanosine, 2-Amino-9-((3aR,4R,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4- yl)-1H-purin-6(9H)-one, C13H17N5O5 CAS NO.362-76-5 MW323.31, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 98.98%, mp163-166°C, Storage: 2-8°C, protect from light, [1]. Anilkumar R Kore, et al. Synthesis and application of a new 2',3'-isopropylidene guanosine substituted cap analog. Bioorg Med Chem Lett. 2008 Sep 1;18(17):4828-32. [2]. H Kasai, et al. Detection and identification of mutagens by the adducts formed upon reaction with guanosine derivatives. IARC Sci Publ. 1986:(70):413-8. [3]. H Kasai, et al. Hydroxylation of guanine in nucleosides and DNA at the C-8 position by heated glucose and oxygen radical-forming agents. Environ Health Perspect. 1986 Aug:67:111-6.

Uridine 5'-monophosphate, 5'-Uridylic acid, C9H13N2O9P CAS NO.58-97-9 MW324.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.98%, Optical Rotation: -5.9°(C=0.01g/mI, H2O, 20°C, 589nm), mp202°C, Storage: Storage temp. -20°C, Uridine 5'-monophosphate (UMP) is the nucleotide monomer in RNA. It contains the nucleobase uracil well as the ribose and phosphate groups. UMP dietary supplementation enhances neurotransmitter release and neurite outgrowth in adult rats and promotes the generation of membrane phospholids. In adult gerbils, oral supplementation with DHA in combination with UMP exhibits an increase in dendritic spines and neuronal membrane synthesis. It proposes strategy to treat cognitive impairment caused by synaptic loss. [1]. Li G, et al. Uridine/UMP metabolism and their function on the gut in segregated early weaned piglets. Food Funct. 2019 Jul 17;10(7):4081-4089. [2]. Aide Lasa, et al. Isolation and identification of Vibrio toranzoniae associated with diseased red conger eel (Genypterus chilensis) farmed in Chile. Vet Microbiol. 2015 Sep 30;179(3-4):327-31. [3]. Christian D Laourdakis, et al. Comprehensive quantitative analysis of purines and pyrimidines in the human malaria parasite using ion-pairing ultra-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Sep 15:967:127-33. [4]. Haitian Fang, et al. Site-directed mutagenesis studies on the uridine monophosphate binding sites of feedback inhibition in carbamoyl phosphate synthetase and effects on cytidine production by Bacillus amyloliquefaciens. Can J Microbiol. 2013 Jun;59(6):374-9. [5]. Imen Lassoued, et al. Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Raja clavata) muscle. J Proteomics. 2015 Oct 14:128:458-68.

6-Chloro-9-[2,3-O-(1-methylethylidene)-beta-D-ribofuranosyl]-9H-Purine, 6-Chloropurine-9-(2,3-isopropylidene-β-D-ribofuranoside), C13H15ClN4O4 CAS NO.39824-26-5 MW326.74, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, mp156-159°C, bp527.8°C at 760 mmHg, Storage: -20°C, sealed storage, away from moisture
-beta-D-ribofuranosyl]-9H-Purine.png)
Cyclic AMP, Cyclic adenosine monophosphate, Adenosine cyclic 3',5'-monophosphate, cAMP, C10H12N5O6P CAS NO.60-92-4 MW329.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.95%, mp260°C, bp701.5°C at 760 mmHg, Storage: Storage temp. -20°C, APPLICATIONS Protein kinase activator. It has the effects of improving myocardial hypoxia, dilating coronary artery, enhancing myocardial contract, and increasing cardiac output. It is used for the adjuvant treatment of angina pectoris and acute myocardial infarction, but its effect lasts for a short time. Occasionally, fever and rash. [1]. Aronoff DM, et, al. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006 Nov;26(11):827-33. [2]. G M Fimia, et al. Cyclic AMP signaling. J Cell Sci. 2001 Jun;114(Pt 11):1971-2. [3]. Paolo Sassone-Corsi, et al. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a011148. [4]. A G Beristain, et al. PKA signaling drives mammary tumorigenesis through Src. Oncogene. 2015 Feb 26;34(9):1160-73. [5]. Adriana María Belén Abiuso, et al. H4 histamine receptors inhibit steroidogenesis and proliferation in Leydig cells. J Endocrinol. 2014 Dec;223(3):241-53.

2'-Deoxyadenosine-5'-monophosphate, C10H14N5O6P CAS NO.653-63-4 MW331.22, form Solid, color White to off-white, Assay: ≥97.0%, mp148°C, bp753.5°C at 760 mmHg, Flash point 409.5°C, Storage: -20°C, protect from light, [1]. Katsuya Narumi, et al. Mutual role of ecto-5'-nucleotidase/CD73 and concentrative nucleoside transporter 3 in the intestinal uptake of dAMP. PLoS One. 2019 Oct 21;14(10):e0223892. [2]. V Duarte, et al. Insertion of dGMP and dAMP during in vitro DNA synthesis opposite an oxidized form of 7,8-dihydro-8-oxoguanine. Nucleic Acids Res. 1999 Jan 15;27(2):496-502. [3]. Hiroshi Sakamaki, et al. Evaluation of column hardware on liquid chromatography-mass spectrometry of phosphorylated compounds. J Chromatogr A. 2015 Feb 13:1381:125-31. [4]. Jérémy Loyau, et al. Robust Antibody-Antigen Complexes Prediction Generated by Combining Sequence Analyses, Mutagenesis, In Vitro Evolution, X-ray Crystallography and In Silico Docking. J Mol Biol. 2015 Aug 14;427(16):2647-62. [5]. Julia Romanova, et al. The effects of addition of mononucleotides on Sma nuc endonuclease activity. ScientificWorldJournal. 2012:2012:454176.
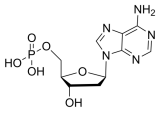
N4-Benzoyl-2'-deoxycytidine, C16H17N3O5 CAS NO.4836-13-9 MW331.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp215-220°C, Storage: Storage temp. 2-8°C
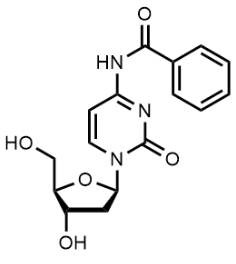
N6-Isopentenyladenosine, Riboprine, C15H21N5O4 CAS NO.7724-76-7 MW335.364, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, mp143-146°C, bp647.2±65.0°C at 760 mmHg, Flash point 345.2±34.3°C, Storage: 2-8°C, protect from light, N6-isopentenyladenosine riboprine is a plant growth hormone. [1]. Cheng HP, et al. Chemical Deprenylation of N6 -Isopentenyladenosine (i6 A) RNA. Angew Chem Int Ed Engl. 2020;59(26):10645-10650. [2]. Colombo F, et al. Pharmacogenomics and analogues of the antitumour agent N6-isopentenyladenosine. Int J Cancer. 2009;124(9):2179-2185. [3]. Ranieri R, et al. N6-isopentenyladenosine dual targeting of AMPK and Rab7 prenylation inhibits melanoma growth through the impairment of autophagic flux. Cell Death Differ. 2018;25(2):353-367.

2',3'-Dideoxy-5-iodouridine, C9H11IN2O4 CAS NO.105784-83-6 MW338.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structur, Assay (LCMS): 97.33%, mp176 °C, Storage: 2-8°C, protect from light, For detecting the virus of tracheitis and treating herpes simplex virus keratitis. [1]. Dinesh Rai, et al. Inhibition of Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium avium by novel dideoxy nucleosides. J Med Chem. 2007 Sep 20;50(19):4766-74. [2]. J M Prober, et al. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987 Oct 16;238(4825):336-41.
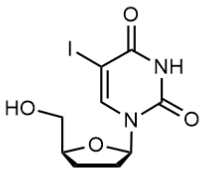
((3aR,4R,6R,6aR)-6-(4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2,2,3a-trimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol, C15H18ClN3O4 CAS NO.2278357-65-4 MW339.77, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Enantiomeric Excess: 100.0%, Assay (HPLC): 99.43%, bp504.5±50.0 °C at 760 mmHg, Storage: -20°C, sealed storage, away from moisture
-6-(4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2,2,3a-trimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol.png)
(3AS,4S,6R,6AR)-6-(6-chloro-purin-9-yl)-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxole-4-carboxylic acid, (3aS,4S,6R,6aR)-6-(6-chloro-9H-purin-9-yl)-2,2-dimethyl-tetrahydrofuro[3,4-d][1,3]dioxole-4-carboxylic acid, C13H13ClN4O5 CAS NO.120355-42-2 MW340.72, form Solid, color White to off-white, LCMS: Consistent with structure, Assay (LCMS): 98.57%, Storage: -20°C, sealed storage, away from moisture
-6-(6-chloro-purin-9-yl)-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxole-4-carboxylic acid.png)
8-Bromoadenosine, C10H12BrN5O4 CAS NO.2946-39-6 MW346.14, form Solid, color Yellow to orange, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.68%, OR[α](C=0.51 g/100ml DMSO): -59.0°, mp210-212°C, bp717.7°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. Man S, et al. Potential and promising anticancer drugs from adenosine and its analogs. Drug Discov Today. 2021 Jun;26(6):1490-1500. [2]. A G Beristain, et al. PKA signaling drives mammary tumorigenesis through Src. Oncogene. 2015 Feb 26;34(9):1160-73. [3]. John J Enyeart, et al. Adrenal fasciculata cells express T-type and rapidly and slowly activating L-type Ca2+ channels that regulate cortisol secretion. Am J Physiol Cell Physiol. 2015 Jun 1;308(11):C899-918. [4]. Jonai Pujol-Giménez, et al. Functional characterization of the human facilitative glucose transporter 12 (GLUT12) by electrophysiological methods. Am J Physiol Cell Physiol. 2015 Jun 15;308(12):C1008-22. [5]. Katherina Katsirntaki, et al. Bronchoalveolar sublineage specification of pluripotent stem cells: effect of dexamethasone plus cAMP-elevating agents and keratinocyte growth factor. Tissue Eng Part A. 2015 Feb;21(3-4):669-82.

8-Bromo-2'-deoxyguanosine, C10H12BrN5O4 CAS NO.13389-03-2 MW346.14, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, mp>250 °C, bp727ºC at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.

5'-Adenylic acid (sodium hydrate), A-5'-P (sodium hydrate), AMP (sodium hydrate), Adenosine 5'-monophosphate (sodium hydrate), C10H14N5O7P.xH2O.xNa CAS NO.149022-20-8, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Storage temp. -20°C, [1]. Giorgia Zadra, et al. New strategies in prostate cancer: targeting lipogenic pathways and the energy sensor AMPK. Clin Cancer Res. 2010 Jul 1;16(13):3322-8. [2]. Makhosazane Zungu, et al. Regulation of AMPK by the ubiquitin proteasome system. Am J Pathol. 2011 Jan;178(1):4-11. [3]. Ming Gan, et al. Extracellular ATP induces intracellular alpha-synuclein accumulation via P2X1 receptor-mediated lysosomal dysfunction. Neurobiol Aging. 2015 Feb;36(2):1209-20. [4]. Misaki Kawanishi, et al. Sensitive and validated LC-MS/MS methods to evaluate mycophenolic acid pharmacokinetics and pharmacodynamics in hematopoietic stem cell transplant patients. Biomed Chromatogr. 2015 Sep;29(9):1309-16. [5]. Tomohito Fujimoto, et al. Identification of a novel CaMKK substrate. Biochem Biophys Res Commun. 2011 Jun 24;410(1):45-51.
.png)
Adenosine monophosphate C10H14N5O7P CAS NO.61-19-8 MW347.22, form Solid, color White to off-white, Assay: ≥98.0%, mp178-185°C, bp798.5°C at 760 mmHg, Flash point 436.7°C, Storage: 2-8°C, protect from light, [1]. Mondal S, et al. Utility of Adenosine Monophosphate Detection System for Monitoring the Activities of Diverse Enzyme Reactions. Assay Drug Dev Technol. 2017 Oct/Nov;15(7):330-341.
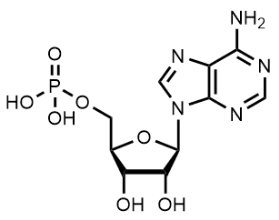
trans-Zeatinriboside, C15H21N5O5 CAS NO.6025-53-2 MW351.36, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.52%, mp176-179°C, bp731.8°C at 760 mmHg, Storage: Storage temp. -20°C, [1]. Osugi A, et al. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat Plants. 2017 Jul 24;3:17112. [2]. Jianchang Yang, et al. Involvement of polyamines in the post-anthesis development of inferior and superior spikelets in rice. Planta. 2008 Jun;228(1):137-49. [3]. Kun Wu, et al. Characterization of a single recessive yield trait mutant with elevated endogenous ABA concentration and deformed grains, spikelets and leaves. Plant Sci. 2011 Feb;180(2):306-12. [4]. Masaru Tanaka, et al. Expression of class I knotted1-like homeobox genes in the storage roots of sweetpotato (Ipomoea batatas). J Plant Physiol. 2008 Nov 1;165(16):1726-35. [5]. Michael Behr, et al. Remodeling of cytokinin metabolism at infection sites of Colletotrichum graminicola on maize leaves. Mol Plant Microbe Interact. 2012 Aug;25(8):1073-82.

Ibacitabine, 5-Iodo-2'-deoxycytidine, C9H12IN3O4 CAS NO.611-53-0 MW353.11, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%OR(C=0.51g/100ml H2O): 16.2°, mp187-189°C, bp522.1°C at 760 mmHg, Storage: 2-8°C, protect from light, [1]. A Serpentier-Daude, Contact dermatitis to topical antiviral drugs. Ann Dermatol Venereol. 2000 Feb;127(2):191-3. [2]. Frank Schoensiegel, et al. MIA (melanoma inhibitory activity) promoter mediated tissue-specific suicide gene therapy of malignant melanoma. Cancer Gene Ther. 2004 Jun;11(6):408-18. [3]. G Smith, et al. In vitro sensitivity of macropodid herpesvirus 2 to selected anti-herpetic compounds. J Wildl Dis. 1996 Jan;32(1):117-20. [4]. J Campisi, et al. Hereditary orotic aciduria, Lesch-Nyhan syndrome, and xeroderma pigmentosum probed by herpes simplex virus: 125I-iododeoxycytidine incorporation as an assay for viral growth. J Cell Physiol. 1983 Jan;114(1):21-8. [5]. R B Van Dyke, et al. Uptake of [125I]iododeoxycytidine by cells infected with herpes simplex virus: a rapid screening test for resistance to acyclovir. J Infect Dis. 1985 Dec;152(6):1206-11.

Idoxuridine, 5-Iodo-2′-deoxyuridine, C9H11IN2O5 CAS NO.54-42-2 MW354.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.70%, mp194°C, Storage: Storage temp. 2-8°C, 5-Iodo-2'-deoxyuridine prevents DNA viral replication in vitro. This has been observed in herpes and pox viruses. It have teratogenic, tumour-promoting, mutagenic and immunosuppressive properties. Topical 5-Iodo-2'-deoxyuridine effective against epithelial infections. [1]. D E Griswold, et al. Stimulation of hemolysin plaque-forming cells by idoxuridine. Cancer Res. 1975 Jan;35(1):88-92. [2]. David J Maggs, et al. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine, and acyclovir against feline herpesvirus type-1. Am J Vet Res. 2004 Apr;65(4):399-403. [3]. Mark N Prichard, et al. Orthopoxvirus targets for the development of antiviral therapies. Curr Drug Targets Infect Disord [4]. Aliuska Morales Helguera, et al. Probing the anticancer activity of nucleoside analogues: a QSAR model approach using an internally consistent training set. J Med Chem. 2007 Apr 5;50(7):1537-45. [5]. Benedetta Arnò, et al. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014 Nov 26:5:5611.
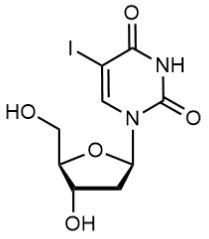
5'-Deoxy-5'-iodouridine, C9H11IN2O5 CAS NO.14259-58-6 MW354.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Storage temp. 2-8°C, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.
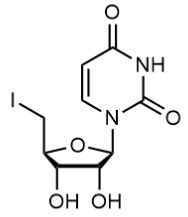
Bz-dA, N6-Benzoyl-2'-deoxyadenosine, C17H17N5O4 CAS NO.4546-72-9 MW355.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 95.79%, mp120-122 °C, Storage: Storage temp. 2-8°C
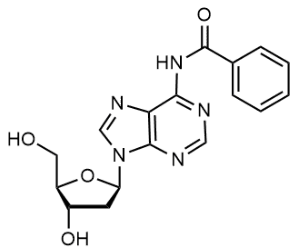
N4-Anisoyl-2'-deoxycytidine, N(sup 4)anisoyl-2'-deoxycytidine, C17H19N3O6 CAS NO.48212-99-3 MW361.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 97.31%, mp175-180 °C, Storage: 2-8°C, stored under nitrogen
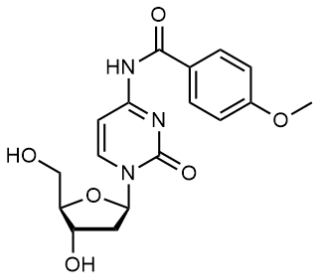
8-Bromoguanosine, C10H12BrN5O5 CAS NO.4016-63-1 MW362.14, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.53%, Optical Rotation[a] -26.001°(C=0.01g/ml DMSO), mp222°C, bp611.1°C at 760 mmHg, Storage: -20°C, protect from light, [1]. Goodman MG, Weigle WO. Intracellular lymphocyte activation and carrier-mediated transport of C8-substituted guanine ribonucleosides. Proc Natl Acad Sci U S A. 1984 Feb;81(3):862-6. [2]. Koo GC, Jewell ME, Manyak CL, Sigal NH, Wicker LS. Activation of murine natural killer cells and macrophages by 8-bromoguanosine. J Immunol. 1988 May 1;140(9):3249-52. [3]. Yajima R, et al. A conformationally restricted guanosine analog reveals the catalytic relevance of three structures of an RNA enzyme. Chem Biol. 2007 Jan;14(1):23-30.

Uridine 5'-monophosphate (disodium salt), 5'-Uridylic acid (disodium salt), C9H11N2Na2O9P CAS NO.3387-36-8 MW368.14, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay: ≥98.0%, mp208-210°C, Storage: 2-8°C, sealed storage, away from moisture, [1]. Hozumi Y, et al. Orotate phosphoribosyltransferase localizes to the Golgi complex and its expression levels affect the sensitivity to anti-cancer drug 5-fluorouracil. Biomed Res. 2015;36(6):403-9.
.png)
5-Iodo-cytidine, 5-Iodo-D-cytidine, C9H12IN3O5 CAS NO.1147-23-5 MW369.11, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=1 g/100ml H2O): -4.9°, Storage: 2-8°C, protect from light, mp>160 °C, bp565.5°C at 760 mmHg, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.

5-Iodouridine, C9H11IN2O6 CAS NO.1024-99-3 MW370.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.44%, OR[α](C=1 g/100ml H2O): -22.1°, mp205-207°C, Storage: 2-8°C, protect from light, 5-iodouridine has been shown to enhance the effect of gamma radiation in hamster cells. Used as a catalyst, petrochemical additive, for organic. [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88. [2]. B L Golden, et al. X-ray crystallography of large RNAs: heavy-atom derivatives by RNA engineering. RNA. 1996 Dec;2(12):1295-305. [3]. Daniel E Ryan, et al. New tertiary constraints between the RNA components of active yeast spliceosomes: a photo-crosslinking study. RNA. 2004 Aug;10(8):1251-65. [4]. K Shah, et al. Synthesis of uridine phosphoramidite analogs: reagents for site-specific incorporation of photoreactive sites into RNA sequences. Bioconjug Chem. 1994 Nov-Dec;5(6):508-12. [5]. Lo?c Ropartz, et al. Phosphine containing oligonucleotides for the development of metallodeoxyribozymes. Chem Commun (Camb). 2007 Apr 21:(15):1556-8.
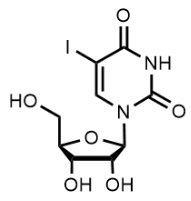
Uridine triacetate, Tri-O-acetyl uridine, C15H18N2O9 CAS NO.4105-38-8 MW370.31, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.40%, Water(KF): 0.2%, mp127.0-131.0 °C, Storage: Storage temp. -20°C, Uridine triacetate (Vistonuridine, PN401) is an orally active prodrug of the natural product nucleosideidine. It is used in the treatment of hereditary orotic aciduria or applied in the treatment of emergencies such as fluorouracil, capecitine overdose or toxicity. [1]. Cada DJ, et al. Uridine Triacetate. Hosp Pharm. 2016 Jun;51(6):484-8. [2]. Ma WW, et al. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer. 2017 Jan 1;123(2):345-356. [3]. Rolando A G Garcia, et al. Prompt treatment with uridine triacetate improves survival and reduces toxicity due to fluorouracil and capecitabine overdose or dihydropyrimidine dehydrogenase deficiency. Toxicol Appl Pharmacol. 2018 Aug 15;353:67-73. [4]. Siennah R Miller, et al. Predicting Drug Interactions with Human Equilibrative Nucleoside Transporters 1 and 2 Using Functional Knockout Cell Lines and Bayesian Modeling. Mol Pharmacol. 2021 Feb;99(2):147-162. [5]. Aleksandra Krstulja, et al. Evaluation of molecularly imprinted polymers using 2',3',5'-tri-O-acyluridines as templates for pyrimidine nucleoside recognition. Anal Bioanal Chem. 2014 Oct;406(25):6275-84.

Bz-rA, N6-Benzoyladenosine, N-(9-(3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide, C17H17N5O5 CAS NO.4546-55-8 MW371.35, form SOlid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 95.94%, OR(C=0.723, DMSO): -46.3°, mp141-145°C, Storage: 2-8°C, protect from light

7-Deaza-2'-deoxy-7-iodoadenosine, 5-(4-amino-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2-(hydroxymethyl)tetrahydrofuran-3-ol, C11H13IN4O3 CAS NO.166247-63-8 MW376.15, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.71%, mp>206 °C, bp657.4±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Frank Seela, et al. Oligonucleotides Containing 7‐Deazaadenines: The Influence of the 7‐Substituent Chain Length and Charge on the Duplex Stability. Research Article

7-Iodo-2',3'-dideoxy-7-deaza-guanosine, C11H13IN4O3 CAS NO.114748-67-3 MW376.15, form Solid, color Off-white to light yellow, MS: Consistent with structure, Assay (HPLC): 98.77%, Storage: 2-8°C, protect from light, [1]. Gene Shen, et al. Thiotriphosphate nucleotide dye terminators for use in nucleic acid sequencing. US20060281100A1.

SAH, S-Adenosylhomocysteine, S-((5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)homocysteine, C14H20N6O5S CAS NO.979-92-0 MW384.41, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp209-211°C, bp787.5°C at 760 mmHg, Flash point 430°C, Storage: -20°C, protect from light

1-((2R,3R,4R)-4-((tert-butyldimethylsilyl)oxy)-3-fluoro-5,5-bis(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, C16H27FN2O6Si CAS NO.1445379-61-2 MW390.48
-4-((tert-butyldimethylsilyl)oxy)-3-fluoro-5,5-bis(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
7-Iodo-7-deaza-2'-deoxyguanosine, 2-amino-7-(4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-4-ol, C11H13IN4O4 CAS NO.172163-62-1 MW392.15, form SOlid, color Off-white to light brown, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.12%, mp>170 °C, Storage: 2-8°C, protect from light, [1]. Ronald Graham, et al. Nucleotide cleavable linkers and uses thereof. WO2020146397A1.

2-Iodoadenosine, C10H12IN5O4 CAS NO.35109-88-7 MW393.14, form Solid, color white to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml NAOH): -53.5°, mp202-203°C, bp776.3°C at 760 mmHg, Flash point 423.3°C, Storage: 2-8°C, protect from light, [1]. Man S, et al. Potential and promising anticancer drugs from adenosine and its analogs. Drug Discov Today. 2021 Jun;26(6):1490-1500.

(2R,3R,4S,5R)-2-(6-Amino-8-iodo-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol, C10H12IN5O4 CAS NO.31281-88-6 MW393.14, form Solid, color Off-white to light brown, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 97.28%, bp730.4±70.0 °C at 760 mmHg, Storage: 2-8°C, protect from light
-2-(6-Amino-8-iodo-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol.png)
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-oxo-1H-purin-9(6H)-yl)tetrahydrofuran-3,4-diyl diacetate (Cytarabine Impurity), C16H18N4O8 CAS NO.3181-38-2 MW394.34, Assay 98%, mp234-236 °C, bp620.7±65.0 °C at 760 mmHg
-2-(Acetoxymethyl)-5-(6-oxo-1H-purin-9(6H)-yl)tetrahydrofuran-3,4-diyl diacetate (Cytarabine Impurity).png)
5'-Guanylic acid (disodium salt), 5'-GMP (disodium salt), 5'-guanosine monophosphate (disodium salt)C10H12N5Na2O8P CAS NO.5550-12-9 MW407.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp300°C, Storage: 2-8°C, sealed storage, away from moisture
.png)
Xanthosine 5'-monophosphate (sodium salt), 5'-Xanthylic acid (sodium salt), C10H11N4Na2O9P CAS NO.25899-70-1 MW408.17, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.58%, mp>116°C, Storage: 2-8°C, sealed storage, away from moisture, [1]. A L Demain, et al. Production of xanthosine-5'-monophosphate and inosine-5'-monophosphate by auxotrophic mutants of a coryneform bacterium. Appl Microbiol. 1965 Sep;13(5):757-61. [2]. Qiong Li, et al. GMP synthase is essential for viability and infectivity of Trypanosoma brucei despite a redundant purine salvage pathway. Mol Microbiol. 2015 Sep;97(5):1006-20. [3]. Sidi A Bencherif, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 2015 Aug 12:6:7556.
.png)
(2R,3S,4S,5R)-2-(Acetoxymethyl)-2-azido-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl diacetate, C15H17N5O9 CAS NO.2459945-87-8 MW411.32

Adenosine 5'-diphosphate, C10H15N5O10P2 CAS NO.58-64-0 MW427.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Storage: 2-8°C, protect from light
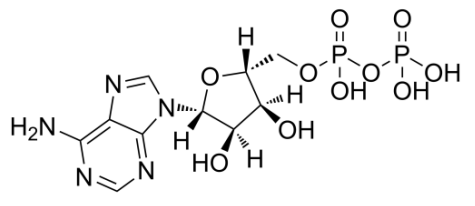
Uridine-5'-diphosphate disodium salt, C9H12N2Na2O12P2 CAS NO.27821-45-0 MW448.12, Storage: -20°C, sealed storage, away from moisture, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: -20°C, sealed storage, away from moisture, [1]. Edyta Gendaszewska-Darmach, et al. Nucleoside 5'-O-monophosphorothioates as modulators of the P2Y14 receptor and mast cell degranulation. Oncotarget. 2016 Oct 25;7(43):69358-69370. [2]. Jacobson KA, et al. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009 Mar;5(1):75-89. [3]. Kim B, et al. Uridine 5'-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol. 2011 Mar 15;186(6):3701-9. [4]. Zainab S B Abbas, et al. UDP-sugars activate P2Y 14 receptors to mediate vasoconstriction of the porcine coronary artery. Vascul Pharmacol. 2018 Apr;103-105:36-46. [5]. Caroline Liot, et al. APC(cdh1) mediates degradation of the oncogenic Rho-GEF Ect2 after mitosis. PLoS One. 2011;6(8):e23676.
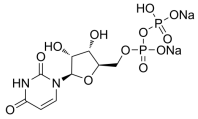
O-Desphenyl Sofosbuvir, Sofosbuvir O-Desphenyl Impurity, C16H25FN3O9P CAS NO.1233335-82-4 MW453.36, form Solid, color Light yellow, Assay: 95.36%, Storage: Storage temp. 2-8°C
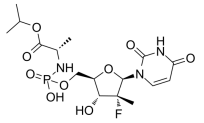
2'-Deoxyguanosine 5'-phosphate tetrahydrate disodium salt, [(2R,3S,5R)-5-(2-amino-6-oxo-3H-purin-9-yl)-3-hydroxyoxolan-2-yl]methyl phosphate, C10H20N5Na2O11P CAS NO.52558-16-4 MW463.25, form Solid, color White to off-white, Assay: ≥99.0%, mp245 °C, Storage: Storage temp. 2-8°C
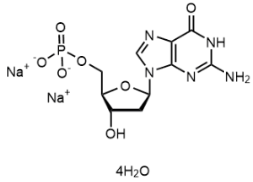
3',5'-Di-O-benzoyl-2'-deoxy-2'-fluoro-2'-methyluridine, RO 2433 Dibenzoate, ((2R,3R,4R,5R)-3-(benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-4- methyltetrahydrofuran-2-yl)methyl benzoate, C24H21FN2O7 CAS NO.863329-65-1 MW468.43, form Solid, color White to off-white, Assay (HPLC): 98.57%, mp256-258 °C, Storage: Storage temp. 2-8°C, RO 2433 Dibenzoate is a related compound of RO 2433 (R637200), a deaminated metabolite of β-D-2'-Deoxy-2'-fluoro-2'-C-methylcytidine, an effective inhibitor of hepatitis C virus (HCV) replication in vitro. [1].Asif, G., et. al.: Antimicrob. Agents Ch., 51, 2877 (2007)
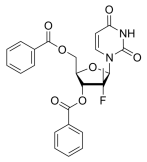
((2S,3S,4S,5S)-3-(benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-4-methyltetrahydrofuran-2-yl)methyl benzoate, C24H21FN2O7 CAS NO.1946820-95-6 MW468.43, Assay: ≥ 97%
-3-(benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-4-methyltetrahydrofuran-2-yl)methyl benzoate.png)
Adenosine 5'-diphosphate (disodium), C10H13N5Na2O10P2 CAS NO.16178-48-6 MW471.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, bp877.7°C at 760 mmHg, Flash point 484.6°C, Storage: -20°C, sealed storage, away from moisture, 5'-diphosphoadenosine (ADP) is an adenine nucleotide that is converted to ATP by ATP synthase and participates energy storage and nucleic acid metabolism. ADP influences platelet activation by interacting with the purinergic receptors P2Y1 and P2Y12. Platelet is inhibited by adenosine receptors after its conversion to adenosine by extracellular-ADPases. [1]. Arts IC, et al. Adenosine 5'-triphosphate (ATP) supplements are not orally bioavailable: a randomized, placebo-controlled cross-over trial in healthy humans. J Int Soc Sports Nutr. 2012 Apr 17;9(1):16. [2]. Hechler B, et al. The P2Y1 receptor is necessary for adenosine 5'-diphosphate-induced platelet aggregation. Blood. 1998 Jul 1;92(1):152-9. [3]. David Erlinge, et al. P2Y receptors in health and disease. Adv Pharmacol. 2011:61:417-39. [4]. David Iyú, et al. Adenosine derived from ADP can contribute to inhibition of platelet aggregation in the presence of a P2Y12 antagonist. Arterioscler Thromb Vasc Biol. 2011 Feb;31(2):416-22. [5]. Lizhen Qiao, et al. A novel surface-confined glucaminium-based ionic liquid stationary phase for hydrophilic interaction/anion-exchange mixed-mode chromatography. J Chromatogr A. 2014 Sep 19:1360:240-7.
.png)
9-((2R,4S,5R)-4-((tert-Butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)tetrahydrofuran-2-yl)-9H-purin-6-amine, C22H41N5O3Si2 CAS NO.51549-32-7 MW479.76, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.74%, Storage: 2-8°C, protect from light
-4-((tert-Butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)tetrahydrofuran-2-yl)-9H-purin-6-amine.png)
((2R,3R,4R,5R)-3-(benzoyloxy)-4-chloro-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-methyltetrahydrofuran-2-yl)methyl benzoate, Sofosbuvir Impurity 17, C24H21ClN2O7 CAS NO.1496551-71-3 MW484.89, Assay 95%, Storage: 2-8°C
-3-(benzoyloxy)-4-chloro-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-methyltetrahydrofuran-2-yl)methyl benzoate.png)
4-Amino-1-[3,5-bis-O-(4-chlorobenzoyl)-2-deoxy-D-erythro-pentofuranosyl]-1,3,5-triazin-2(1H)-one, C22H18Cl2N4O6 CAS NO.1193165-26-2 MW505.31, Assya 98%, bp642.6±65.0 °C at 760 mmHg, Storage: storage temp. 2-8°C, sealed storage, away from moisture
-2-deoxy-D-erythro-pentofuranosyl]-1,3,5-triazin-2(1H)-one.png)
Cytidine-5'-triphosphate (disodium), Cytidine triphosphate (disodium), 5'-CTP (disodium), C9H14N3Na2O14P3 CAS NO.36051-68-0 MW527.12, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.12%, bp849.2°C at 760 mmHg, Flash point 467.4°C, Storage: -20°C, sealed storage, away from moisture, [1]. Kazuyuki Takai, et al. Practical cell-free protein synthesis system using purified wheat embryos. Nat Protoc. 2010 Feb;5(2):227-38. [2]. Xi Liu, et al. LC-based targeted metabolomics analysis of nucleotides and identification of biomarkers associated with chemotherapeutic drugs in cultured cell models. Anticancer Drugs. 2014 Jul;25(6):690-703.
.png)
1-(3,5-Di-O-p-chlorobenzoyl-2-deoxy-β-D-ribofuranosyl)-5-ethyluracil, 1-(3,5-Di-O-p-chlorobenzoyl-2-deoxy-β-D-ribofuranosyl)-5-ethyluracil, Uridine 2'-deoxy-5-ethyl-3',5'-bis(p-chlorobenzoate), [3-(4-chlorobenzoyloxy)-5-(5-ethyl-4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methyl 4-chlorobenzoate, C25H22Cl2N2O7 CAS NO.25137-84-2 MW533.36, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.70%, Water(KF): 0.02%, Residue on Ignition: 0.01%, Loss on drying: 0.10%, Storage: Storage temp. 2-8°C,
-5-ethyluracil.png)
dUTP (trisodium), 2'-Deoxyuridine-5'-triphosphate (trisodium salt), C9H12N2Na3O14P3 CAS NO.102814-08-4 MW534.09, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: 2-8°C, sealed storage, away from moisture, [1]. Nayun Kim, et al. dUTP incorporation into genomic DNA is linked to transcription in yeast. Nature. 2009 Jun 25;459(7250):1150-3. [2]. A Kalogeraki, et al. Apoptosis and cell proliferation correlated with tumor grade in patients with lung adenocarcinoma. In Vivo. 2010 Sep-Oct;24(5):667-70. [3]. Claudia Ulbrich, et al. The impact of vascular endothelial growth factor and basic fibroblast growth factor on cardiac fibroblasts grown under altered gravity conditions. Cell Physiol Biochem. 2010;26(6):1011-22. [4]. Indalecio Quesada-Soriano, et al. Kinetic properties and specificity of trimeric Plasmodium falciparum and human dUTPases. Biochimie. 2010 Feb;92(2):178-86. [5]. Kwou-Yeung Wu, et al. Mechanism of mitomycin-induced apoptosis in cultured corneal endothelial cells. Mol Vis. 2008 Sep 15:14:1705-12.
.png)
Uridine triphosphate (trisodium salt),Uridine 5'-triphosphate trisodium salt, UTP trisodium salt, C9H12N2Na3O15P3 CAS NO.19817-92-6 MW550.09, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, mp140°C, Storage: -20°C, sealed storage, away from moisture, [1]. Choi JH, et al. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas. 2013 May;42(4):680-6.
.png)
Sofosbuvir Impurity 4, N4,3',5'-Tribenzoyl,2'-deoxy-2'-methylene Cytidine, 5-(4-(benzoylimino)-2-hydroxypyrimidin-1(4H)-yl)-2-((benzoyloxy)methyl)-4-methylenetetrahydrofuran-3-yl benzoate, (2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-methylenetetrahydrofuran-3-yl benzoate, C31H25N3O7 CAS NO.863329-63-9 MW551.55, form Solid, color Light yellow to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp173.4-174.4 °C, Storage: Storage temp. 2-8°C

5'-O-(4,4'-Dimethoxytrityl)-2'-O-methyluridine, 1-((2R,3R,4R,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-3-methoxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, 1-(5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-3-methoxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, C31H32N2O8 CAS NO.103285-22-9 MW560.59, form Solid, color Light yellow to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 95.15%, Storage: Storage temp. 2-8°C, [1]. Connolly GP, et al. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci. 1999 May;20(5):218-25.
-2'-O-methyluridine.png)
(2R,3R,4S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-hydroxy-4-methyltetrahydrofuran-3-yl benzoate, Sofosbuvir Impurity 12, C31H27N3O8 CAS NO.863329-62-8 MW569.56, Assay 98%, Storage: Storage temp. 2-8°C
-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-hydroxy-4-methyltetrahydrofuran-3-yl benzoate.png)
Bz-PSI-6130, 5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate, (2R,3R,4R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate, C31H26FN3O7 CAS NO.817204-32-3 MW571.55, form Solid, color White to off-white, Assay (HPLC): 99.06%, mp241°C, Storage: 2-8°C, protect from light
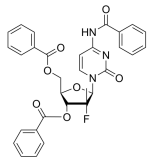
(2R,3R,4R,5S)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate, (2R,3R,4S,5S)-5-(4-(benzoylimino)-2-hydroxypyrimidin-1(4H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate, Sofosbuvir Impurity X, C31H26FN3O7 CAS NO.874638-94-5 MW571.55, Assay 97%, mp174-175 °C, Storage: 2-8°C, protect from light
-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate.png)
(2S,3S,4S,5S)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate, C31H26FN3O7 MW571.55, Assay (NMR): 95%, Storage: Store at room temperature
-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate.png)
5'-O-DMT-N4-Ac-dC, N4-Acetyl-2'-deoxy-5'-O-DMT-cytidine, N-(1-(5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxytetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)acetimidic acid, N-(1-((2R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxytetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)acetamide, C32H33N3O7 CAS NO.100898-63-3 MW571.62, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C, [1]. Dohno C, et, al. Amphiphilic DNA Duplex Stabilized by a Hydrophobic Zipper. European Journal of Organic Chemistry. September 2012. 27: 5317-5323.
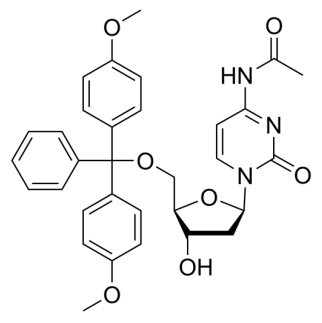
(2R,3R,4R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-chloro-4-methyltetrahydrofuran-3-yl benzoate, Sofosbuvir Impurity E, C31H26ClN3O7 CAS NO.1496551-70-2 588.01, Assay 98%, Storage: Storage temp. 2-8°C,
-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-chloro-4-methyltetrahydrofuran-3-yl benzoate.png)
(2R,3S,4R,5R)-2-azido-2-((benzoyloxy)methyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl dibenzoate, Uridine 4'-C-azido-2',3',5'-tribenzoate, C30H23N5O9 CAS NO.1365255-42-0 MW597.53, Assay 95%, Storage: -20°C, stored under nitrogen
-2-azido-2-((benzoyloxy)methyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl dibenzoate.png)
(2S,3S,4R,5R)-2-azido-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-(iodomethyl)tetrahydrofuran-3,4-diyl dibenzoate, Uridine 4'-C-azido-5'-deoxy-5'-iodo-2',3'-dibenzoate, C23H18IN5O7 CAS NO.139419-02-6 MW603.32, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: -20°C, stored under nitrogen
-2-azido-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-(iodomethyl)tetrahydrofuran-3,4-diyl dibenzoate.png)
1-((2R,3R,4R,5R)-5-((Bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-3-(2-methoxyethoxy)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione, 5'-O-DMTr- 2'-O-(2-Methoxyethyl)-uridine, C33H36N2O9 CAS NO.251647-51-5 MW604.65, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 96.21%, Storage: 2-8°C, protect from light
-5-((Bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-3-(2-methoxyethoxy)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione.png)
5'-O-DMT-N2-ibu-dG, N2-Isobutyryl-5′-O-(4,4′-dimethoxytrityl)-2′-deoxyguanosine, C35H37N5O7 CAS NO.68892-41-1 MW639.70, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp150°C, bp671.92 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Meinolf Lange, et al. Synthesis of oligonucleotides. WO2008141682A1.
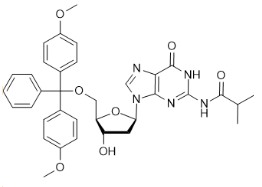
4-amino-1-((2R,3R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(chloromethyl)-3-fluorotetrahydrofuran-2-yl)pyrimidin-2(1H)-one, C32H45ClFN3O4Si2 CAS NO.1445379-90-7 MW646.34, Assay 98%, Storage: Storage temp. 2-8°C,
-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(chloromethyl)-3-fluorotetrahydrofuran-2-yl)pyrimidin-2(1H)-one.png)
(2R,3S,4R,5R)-2-Azido-2-((benzoyloxy)methyl)-5-(2-oxo-4-(1H-1,2,4-triazol-1-yl)pyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl dibenzoate, C32H24N8O8 MW648.58, Assay 95%, Storage: -20°C, stored under nitrogen
-2-Azido-2-((benzoyloxy)methyl)-5-(2-oxo-4-(1H-1,2,4-triazol-1-yl)pyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl dibenzoate.png)
5'-O-DMT-Bz-rA, N-(9-(5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide, N-(9-((2R,3R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide, C38H35N5O7 CAS NO.81246-82-4 MW673.71, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.45%, OR[α](C=0.529 g/100ml MEOH): -23.9°, Storage: Storage temp. 2-8°C, [1]. Boyu Zhong, et al. Cyclic di-nucleotide compounds and methods of use. Patent WO2017161349.

N-(1-((2R,3R,4R,5R)-5-((bis(4-Methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-3-(2-methoxyethoxy)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)benzamide, N-Benzoyl-5'-O-Dmtr-2'-O-(2-Methoxyethyl)-Cytidine, C40H41N3O9 CAS NO.251647-49-1 MW707.77, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, Storage: 2-8°C, protect from light,
-5-((bis(4-Methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-3-(2-methoxyethoxy)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)benzamide.png)
(2R,3R,4R,5R)-2-((benzoyloxy)methyl)-5-(4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)tetrahydrofuran-3,4-diyl dibenzoate, C32H23ClIN3O7 CAS NO.480439-89-2 MW723.90, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: -20°C, stored under nitrogen
-2-((benzoyloxy)methyl)-5-(4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)tetrahydrofuran-3,4-diyl dibenzoate.png)
N-Benzoyl-5'-O-dmtr-2'-O-(2-methoxyethyl)-adenosine, C41H41N5O8 CAS NO.251647-48-0 MW731.79, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.85%, Storage: Storage temp. 2-8°C
-adenosine.png)
((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl ((R)-2-((tert-butoxycarbonyl)amino)-5-oxo-5-(tritylamino)pentanoyl)sulfamate, C42H48N8O10S MW856.94, Assay 95%, Storage: Storage temp. 2-8°C
-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl ((R)-2-((tert-butoxycarbonyl)amino)-5-oxo-5-(tritylamino)pentanoyl)sulfamate.png)
DMT-2'fluoro-da(bz) amidite, C47H51FN7O7P CAS NO.136834-22-5 MW875.92, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Storage temp. -20°C
 amidite.png)
DMT-2'O-Methyl-rC(tac) phosphoramidite, C52H64N5O10P CAS NO.179486-26-1 MW950.07, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: 2-8°C, stored under nitrogen
 phosphoramidite.png)
2'-Deoxyguanosine hydrate, C10H13N5O4.H2O CAS NO.207121-55-9 MW267.24(anhydrous basis), mp>300 °C, form Solid, color, White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: 2-8°C, protect from light
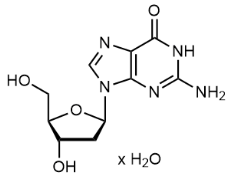
3′-Deoxyuridine, C9H12N2O5 CAS NO.7057-27-4 MW228.20, form Solid, color White to light yellow, Assay (HPLC): 96.22%, mp176-177°C, Storage: 2-8°C, protect from light, [1]. Lin TS, et al. Synthesis and anticancer activity of various 3'-deoxy pyrimidine nucleoside analogues and crystal structure of 1-(3-deoxy-beta-D-threo-pentofuranosyl)cytosine. J Med Chem. 1991;34(2):693-701.

1-((2R,4S,5S)-4-Amino-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione, 3'-Aminothymidine, C10H15N3O4 CAS NO.52450-18-7 MW241.24, Assay 98%, mp161-162 °C,
-4-Amino-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione.png)
2'-Amino-2'-deoxyuridine, C9H13N3O5 CAS NO.26889-39-4 MW243.22, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 97.34%, mp196-198°C, Storage: 2-8°C, protect from light
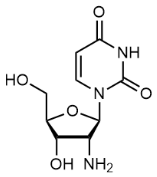
2-Amino-9-((2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-purin-6(9H)-one, 2',3'-Dideoxyguanosine, C10H13N5O3 CAS NO.85326-06-3 MW251.24, Assay 98%, bp632.1 °C at 760 mmHg, Storage: 2-8°C,
-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-purin-6(9H)-one.png)
2-Amino-9-((2R,4S,5S)-4-amino-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-purin-6(9H)-one, 3'-Amino-2',3'-dideoxyguanosine, C10H14N6O3 CAS NO.66323-49-7 MW266.26, Assay 97%,
-4-amino-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-purin-6(9H)-one.png)
2'-Amino-2'-deoxyadenosine, 9-(2-Amino-2-deoxypentofuranosyl)-9H-purin-6-amine, C10H14N6O3 CAS NO.10414-81-0 MW266.26, mp190-191°C, Assay 98%, bp657.6°C at 760 mmHg, Flash point 351.5°C, Storage: 2-8°C,

2-Ethoxy-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidin-4(1H)-one, 2-O-Ethylthymidine, C12H18N2O5 CAS NO.59495-21-5 MW270.28, Assay 97%, bp452.8±55.0 °C at 760 mmHg, 2-O-Ethylthymidine is a compound that can be used for organic synthesis. Building Blocks, Nucleic Acid
-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidin-4(1H)-one.png)
5'-Amino-5'-deoxyguanosine, 2-amino-9-((2R,3R,4S,5R)-5-(aminomethyl)-3,4-dihydroxytetrahydrofuran-2-yl)-1H-purin-6(9H)-one, C10H14N6O4 CAS NO.4099-84-7 MW282.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: -20°C, stored under nitrogen

2'-O-Methylinosine, C11H14N4O5 CAS NO.3881-21-8 MW282.26, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, mp153-156 °C, bp724.3±60.0 °C at 760 mmHg, Storage: 2-8°C, stored under nitrogen, 2'-O-Methylinosine is a novel nucleoside, which is a component of Crithidia fasciculata rRNA. Moreover, it has intrinsic antihypertensive activity is also a nucleoside that induces apoptosis (AINs). [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.
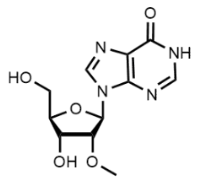
2',3'-O-Isopropylideneuridine, 2',3'-O-Isopropylidene-D-uridine, C12H16N2O6 CAS NO.362-43-6 MW284.27, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.02%, Optical Rotation[a]: -19°(C=0.01g/ml MEOH), mp163-166°C, bp426.76 °C at 760 mmHg, Storage: Storage temp. -20°C , [1]. Anita R Maguire, et al. New methods for the synthesis of N-benzoylated uridine and thymidine derivatives; a convenient method for N-debenzoylation. Carbohydr Res. 2002 Feb 18;337(4):369-72. [2]. C S Yao, et al. Synthesis and central nervous system depressant effects of N3-substituted 2',3'-O-isopropylideneuridines. Chem Pharm Bull (Tokyo). 1999 Dec;47(12):1802-4. [3]. M L Scholes, et al. Enhancement of radiation-induced base release from nucleosides in alkaline solution: essential role of the O.- radical. Int J Radiat Biol. 1992 Apr;61(4):443-9. [4]. Marietta Tóth, et al. 5'-Uridyl derivatives of N-glycosyl allophanic acid and biuret. Carbohydr Res. 2010 Jan 11;345(1):163-7. [5]. Tomomi Shimizu, et al. Synthesis of N3-substituted uridine and related pyrimidine nucleosides and their antinociceptive effects in mice. Chem Pharm Bull (Tokyo). 2005 Mar;53(3):313-8.

2′-Deoxy-2′-fluoroguanosine, C10H12FN5O4 CAS NO.78842-13-4 MW285.23, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.67%, mp262-264°C, Storage: 2-8°C, protect from light, 2′-Deoxy-2′-fluoroguanosine is an influenza virus polymerase inhibitor, which, like ribavirin, is used as an antiviral drug against avian (H5N) influenza viruses. [1]. Jakeman KJ, et, al. Efficacy of 2'-deoxy-2'-fluororibosides against influenza A and B viruses in ferrets. Antimicrob Agents Chemother. 1994 Aug;38(8):1864-7. [2]. Tisdale M, et, al. Inhibition of influenza virus transcription by 2'-deoxy-2'-fluoroguanosine. Antimicrob Agents Chemother. 1995 Nov;39(11):2454-8.

(2R,3R,5R)-5-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-4,4-difluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol, (2R,3R,5R)-5-{4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-4,4-difluoro-2-(hydroxymethyl)oxolan-3-ol, C11H12F2N4O3 CAS NO.156058-35-4 MW286.23, Assay 95%, bp584.2±50.0 °C at 760 mmHg
-5-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-4,4-difluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol.png)
8-Mercaptoadenosine, 8-Thioadenosine, C10H13N5O4S CAS NO.3001-45-4 MW 299.31, Assay 97%,
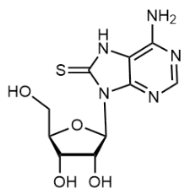
6-Chloroguanineriboside, 6-Chloroguanosine, 2-Amino-6-chloropurine Riboside, C10H12ClN5O4 CAS NO.2004-07-1 MW301.69, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=0.61g/100ml H2O): -30.1°, mp165-167°C, bp729.9°C at 760 mmHg, Storage: -20°C, sealed storage, away from moisture and light, It is a new type of endogenous protein degradation inhibitor. [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88. [2]. A Matsuda, et al. Synthesis of a mutagenic nucleoside, 2'-deoxy-2-(p-nitrophenyl)-adenosine. Nucleic Acids Symp Ser. 1986:(17):141-3. [3]. J T Kusmierek, et al. Preparative electrochemical reduction of 2-amino-6-chloropurine and synthesis of 6-deoxyacyclovir, a fluorescent substrate of xanthine oxidase and a prodrug of acyclovir. Acta Chem Scand B. 1987 Nov;41(10):701-7.

Xanthosine (dihydrate), C10H16N4O8 CAS NO.5968-90-1 MW320.26, form Solid, color White to Off-White, Assay 98%(HPLC), mp>215 °C, Solubility: Aqueous Base (Slightly), DMSO (Slightly), Methanol (Slightly), Storage: -20°C, Xanthine plays a key role in purine metabolism. High levels of xanthine excretion have been observed in breast cancer. The deamination product of Guanosine. Potential biomarker for detecting radiation exposure. Used in the amplification of DNA isothermal strand displacement. [1]. Choudhary S, et, al. Examination of the xanthosine response on gene expression of mammary epithelial cells using RNA-seq technology. J Anim Sci Technol. 2018 Jul 13;60:18. [2]. Lucas Willmann, et al. Metabolome analysis via comprehensive two-dimensional liquid chromatography: identification of modified nucleosides from RNA metabolism. Anal Bioanal Chem. 2015 May;407(13):3555-66. [3]Magasanik, B, et al.: J. Bio. Chem., 206, 83 (1954).
.png)
9-((3aR,4R,6R,6aR)-2,2-Dimethyl-6-((methylamino)methyl)tetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-amine, C14H20N6O3 CAS NO.34245-49-3 MW320.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥96.0%, Storage: 2-8°C, protect from light
-2,2-Dimethyl-6-((methylamino)methyl)tetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-amine.png)
5-Bromouridine, C9H11BrN2O6 CAS NO.957-75-5 MW323.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00g/100ml H2O): -2.2°, mp180-182°C, solubility: Water (Sonicated), Storage: Storage temp. -20°C, 5-Bromo-uracil nucleoside is usually incorporated into RNA for detection by immunocytochemistry and cell counting analysis. It has antiral activity and inhibits the activity of viruses such as human immunodeficiency virus. [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88. [2].Kramer, K., et al.: Int. J. Mass. Spect., 304, 184 (2011); Denifi, S., et al.: Euro. Phys. J. Atomic. Molec. Optical. Phys., 35, 391 (2005);

2',3'-Dideoxy-5-iodocytidine, 4-Amino-1-((2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-iodopyrimidin-2(1H)-one, C9H12IN3O3 CAS NO.114748-57-1 MW337.11, Assay 98%, mp>156 °C, bp448.2±55.0 °C at 760 mmHg, Storage: -20 °C, It can be used as an antibiotic, especially effective against mycobacteria. [1]. Dinesh Rai, et al. Inhibition of Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium avium by novel dideoxy nucleosides. J Med Chem. 2007 Sep 20;50(19):4766-74.
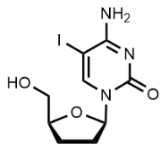
iBu-dG, N2-Isobutyryl-2'-deoxyguanosine, C14H19N5O5 CAS NO.68892-42-2 MW337.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥99.0%, mp≥300°C, Storage: Storage temp. 2-8°C, 2'-Deoxy-N-(2-methyl-1-oxopropyl)-guanosine is used for the preparation of dimethylcarbamate dinucleotides, as HIV-1 nucleocapsid protein NCp7 inhibitors.
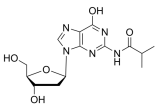
8-Bromoinsine, 8-Bromoinosine, 8-Bromo-9-β-D-ribofuranosyl-6H-purin-6-one, C10H11BrN4O5 CAS NO.55627-73-1 MW347.12, form Solid, color White to yellow, Assay 97%, mp180-190 °C, Storage: storage temp. 2-8°C
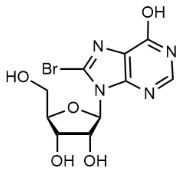
N-Benzoylcytidine,N4-Benzoylcytidine, C16H17N3O6 CAS NO.13089-48-0 MW347.327, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp230-234°C, Solubility: 4.9 mg/mL in DMSO, 5.3mg/mL in Dimethylformamide(sonicated), Not soluble in Me, Storage: Storage temp. 2-8°C, Building Blocks; Nucleic Acid; A useful building block for oligoribonucleotide synthesis. [1]. Shestopalov, I., et al.: Nat. Chem. Biol., 3, 650 (2007),

iBu-rG, N-Isobutyrylguanosine, C14H19N5O6 CAS NO.64350-24-9 MW353.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=0.50g/100ml ETOH): -11.0°, mp148 °C, solubility: DMSO (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, N-Isobutyrylguanosine was used to synthesize 2'-O-(o-nitrobenzyl)-3'-ioguanosine phosphoramidite, which was then used to form oligonucleotides, a substrate used to explore the RNA catalytic mechanism, [1]. Li, N., et. al.: J. Org. Chem., 79, 3647 (2014)
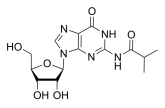
((2R,3S,4R,5R)-5-(4-Amino-3-methyl-2-oxo-2,3-dihydropyrimidin-1(6H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl methyl sulfate, 3-Methyl Cytidine Methosulfate, 3-Methylcytidine Mono(Methyl Sulfate) Salt, C11H19N3O8S CAS NO.21028-20-6 MW353.35, form Solid, color White, MS Conforms to Structure, NMR Conforms to Structure, Assay (HPLC): 99%, mp230-233 °C, solubility: DMSO (Slightly), Methanol (Slightly), Storage: -20°C, Standards; Nucleic Acids; Pharmaceutical/API Drug Impurities/Metabolites; Cytidine derivative., [1]. Ueda, T., et al.: Chem. Pharmaceut. Bull., 22, 2377 (1974),
-5-(4-Amino-3-methyl-2-oxo-2,3-dihydropyrimidin-1(6H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl methyl sulfate.png)
3'-(Benzylamino)-3'-deoxyadenosine, (2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-4-(benzylamino)-5-(hydroxymethyl)tetrahydrofuran-3-ol, C17H20N6O3 CAS NO.67313-10-4 MW356.38, Assay 97%,
-3'-deoxyadenosine.png)
3'-O-(t-Butyldimethylsilyl)-2'-deoxy-2'-fluorouridine, 2'-Deoxy-3'-O-[(1,1-dimethylethyl)dimethylsilyl]-2'-fluorouridine, C15H25FN2O5Si CAS NO.1445379-59-8 MW360.45, form Solid, color White to Off-White, mp182-185 °C, solubility: Chloroform (Slightly, Sonicated), DMSO (Slightly), Methanol (Slightly, Sonicated), Storage: 2-8°C, Building Blocks; Chiral Molecules; 2'-Deoxy-3'-O-[(1,1-Dimethylethyl)Dimethylsilyl]-2'-Fluorouridine is a useful compound for the synthetic preparation of 4'-halogen containing nucleotide and nucleoside therapeutics. [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88. [2]. Painter, G. R., et al.: PCT Int. Appl. (2019), WO 2019173602 A1
-2'-deoxy-2'-fluorouridine.png)
N-(1-((2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)benzamide, C17H18FN3O5 CAS NO.874638-98-9 MW363.34, Assay 98%, Storage: 2-8°C
-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)benzamide.png)
2'-Deoxyguanosine 5'-monophosphate (disodium), 5′-dGMP (disodium), C10H12N5Na2O7P CAS NO.33430-61-4 MW391.18, Assay 98%, mp>245°C, Storage: 2-8°C, sealed storage, away from moisture and light, [1]. M Paula Denofrio, et al. The photosensitizing activity of lumazine using 2'-deoxyguanosine 5'-monophosphate and HeLa cells as targets. Photochem Photobiol Sci. 2009 Nov;8(11):1539-49.
.png)
2',3',5'-Tri-O-acetyl adenosine, Adenosine 2',3',5'-triacetate, C16H19N5O7CAS NO.7387-57-7 MW393.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.55%, Optical Rotation[a]: -30.7°(C=0.007101g/ml CHCL3), mp168-170 °C, bp594.1°C at 760 mmHg, Flash point 313.1°C, solubility: Chloroform (Sparingly), DMSO (Slightly), Methanol (Sparingly), Storage: -20°C, protect from light, [1]. Man S, et al. Potential and promising anticancer drugs from adenosine and its analogs. Drug Discov Today. 2021 Jun;26(6):1490-1500. [2]. M Watanabe, et al. Detection of proton-acceptor sites of hydrogen bonding in adenine X uracil base pairs by the use of 15N magnetic resonance. Eur J Biochem. 1981 Jul;117(3):553-8.
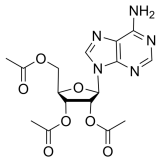
(2R,3R,4S,5S,6R)-2-((6-(Benzylamino)-9H-purin-9-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, 6-Benzylaminopurine 9-(b-D-glucoside), N6-Benzyladenine 9-Glucoside, C18H21N5O5, CAS NO.4294-17-1 MW387.39, form Solid, color Off-White to Pale Yellow, NMR Conforms to Structure, MS Conforms to Structure, Assay (HPLC): 96.57%, mp179 °C, Solubility: DMSO (Slightly), Methanol(Slightly), Storage: 2-8℃,, protect from light, Useful in organic synthesis.
-2-((6-(Benzylamino)-9H-purin-9-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.png)
2′,3′,5′-Tri-O-acetyl-6-chloroguanosine,2-Amino-6-chloro-9-(2,3,5-tri-O-acetyl-Beta-D-ribofuranosyl)purine, C16H18ClN5O7 CAS NO.16321-99-6 MW427.80, form SOlid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.13%, mp146.0-150.0 °C, bp637 °C at 760 mmHg, Storage: -20°C, sealed storage, away from moisture and light, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.

2'-Deoxycytidine-5'-diphosphate (trisodium), dCDP (trisodium), C9H12N3Na3O10P2 CAS NO.151151-32-5 MW453.12, Assay 98%, Storage: -80°C, sealed storage, away from moisture and light, [1]. Dimitri Topalis, et al. Looking for new pyrimidine acyclic nucleotide analogues designed for phosphorylation by human UMP-CMP kinase. Nucleosides Nucleotides Nucleic Acids. 2007;26(10-12):1369-73. [2]. Hüseyin Besir, et al. Structure of a halophilic nucleoside diphosphate kinase from Halobacterium salinarum. FEBS Lett. 2005 Dec 5;579(29):6595-600. [3]. Mahmoud Kandeel, et al. Substrate specificity and nucleotides binding properties of NM23H2/nucleoside diphosphate kinase homolog from Plasmodium falciparum. J Bioenerg Biomembr. 2010 Oct;42(5):361-9.
.png)
((2R,3R,4S,5R)-3-(benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-4-methyltetrahydrofuran-2-yl)methyl benzoate, C24H22N2O8 CAS NO.1910099-11-4 MW466.44, Assay 97%
-3-(benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-4-methyltetrahydrofuran-2-yl)methyl benzoate.png)
Inosine-5'-diphosphoric acid (disodium), Inosine-5-diphosphoric Acid Disodium Salt, C10H12N4Na2O11P2 CAS NO.54735-61-4 MW472.15, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥97.0%, bp513.3°C at 760 mmHg, Storage: 2-8°C, sealed storage, away from moisture and light

2'-Deoxyguanosine-5'-diphosphate trisodium salt, C10H12N5Na3O10P2 CAS NO.102783-74-4 MW493.15, form Solid, color, White to off-white (Solid), Assay: ≥98.0%, Water(KF): 6.126%, solubility: Aqueous Base (Slightly), Water (Slightly), mp>178 °C, Storage: -20°C, protect from light, 2'-deoxyguanosine 5'-diphosphate (dGDP) inhibits xanthine phosphoribosyltransferase (XT) and hypoxanthine-guanine phosphoribosyltransferase, 2'-Deoxyguanosine-5'-diphosphate is a deoxyguanosine kinase inhibitor [1]. Jiande Gu, et al. Electron attachment to DNA single strands: gas phase and aqueous solution. Nucleic Acids Res. 2007;35(15):5165-72. [2]. Mahmoud Kandeel, et al. Substrate specificity and nucleotides binding properties of NM23H2/nucleoside diphosphate kinase homolog from Plasmodium falciparum. J Bioenerg Biomembr. 2010 Oct;42(5):361-9. [3]. Ngoc Q Tran, et al. A novel nucleotide kinase encoded by gene 1.7 of bacteriophage T7. Mol Microbiol. 2010 Jul;77(2):492-504. [4]. Ngoc Q Tran, et al. Gene 1.7 of bacteriophage T7 confers sensitivity of phage growth to dideoxythymidine. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9373-8. [5]. Dayer, Mohammad R., et al.: Res. J. of Veterinary Sci., 5(1), 1-12 (2012).

8-Bromo-5'-O-(4-cyanobenzyl)-2',3'-di-O-isopropylidene adenosine, C21H21BrN6O4 CAS NO.1134156-53-8 MW501.33, Assay 98%, bp712.8±70.0°C at 760 mmHg, Flash point 384.9±35.7°C, Storage: 2-8°C, [1]. Man S, et al. Potential and promising anticancer drugs from adenosine and its analogs. Drug Discov Today. 2021 Jun;26(6):1490-1500.
-2',3'-di-O-isopropylidene adenosine.png)
tert-Butyl N-{9-[(3aR,4R,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyl-tetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl]-9H-purin-6-yl}-N-[(tert-butoxy)carbonyl]carbamate, C23H33N5O8 CAS NO.1152172-19-4 MW507.54, Assay 98%, bp626.3±65.0 °C at 760 mmHg, As a reactant in the synthesis of inhibitors of mycobacterium tuberculosis iron carrier biosynthesis.
-6-(hydroxymethyl)-2,2-dimethyl-tetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl]-9H-purin-6-yl}-N-[(tert-butoxy)carbonyl]carbamate.png)
2'-Deoxyadenosine 5'-triphosphate (disodium), 5'-Dexoadenosine triphosphate (disodium), C10H14N5Na2O12P3 CAS NO.74299-50-6 MW535.15, Assay 98%, Storage: -20°C, [1]. J E Reardon, et al. Human immunodeficiency virus reverse transcriptase: steady-state and pre-steady-state kinetics of nucleotide incorporation. Biochemistry. 1992 May 12;31(18):4473-9. [2]. J F Burd, et al. Effect of incubation conditions on the nucleotide sequence of DNA products of unprimed DNA polymerase reactions. J Mol Biol. 1970 Nov 14;53(3):435-59. [3]. Jennifer N Patro, et al. Role of the 2-amino group of purines during dNTP polymerization by human DNA polymerase alpha. Biochemistry. 2009 Jan 13;48(1):180-9. [4]. Michael Trostler, et al. Discrimination between right and wrong purine dNTPs by DNA polymerase I from Bacillus stearothermophilus. Biochemistry. 2009 Jun 2;48(21):4633-41. [5]. W M Kati, et al. Mechanism and fidelity of HIV reverse transcriptase. J Biol Chem. 1992 Dec 25;267(36):25988-97.
.png)
5'-O-[Bis(4-methoxyphenyl)phenylmethyl]-2'-deoxy-2'-fluoro-5-methyluridine, 2'-Deoxy-2'-fluoro-5'-O-(4,4'-dimethoxytrityl)-5-methyluridine, C31H31FN2O7 CAS NO.133324-02-4 MW562.59, Assay 96%
phenylmethyl]-2'-deoxy-2'-fluoro-5-methyluridine.png)
Deoxyguanosine triphosphate (trisodium) solution (100mM), dGTP (trisodium) solution (100mM), 2'-Deoxyguanosine-5'-triphosphate (trisodium) solution (100mM), C10H13N5Na3O13P3 CAS NO.93919-41-6 MW573.13, form Liquid, color Colorless to light yellow, Assay: ≥99.0%, Storage: Solution, -20°C, 2 years, [1]. Marco Gatti, et al. The Ubiquitin Ligase TRIP12 Limits PARP1 Trapping and Constrains PARP Inhibitor Efficiency. Cell Rep. 2020 Aug 4;32(5):107985.
 solution (100mM).png)
N-(1-((6aR,8R,9S,9aR)-9-hydroxy-2,2,4,4-tetraisopropyl-9-methyltetrahydro-6H-furo[3,2-f][1,3,5,2,4]trioxadisilocin-8-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)benzamide, C29H45N3O7Si2 CAS NO.817204-34-5 MW603.85, Assay 97%, Storage: 2-8°C
-9-hydroxy-2,2,4,4-tetraisopropyl-9-methyltetrahydro-6H-furo[3,2-f][1,3,5,2,4]trioxadisilocin-8-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)benzamide.png)
(2R,3S,5R)-5-(5-((E)-2-bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-(((4-chlorobenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-chlorobenzoate, C25H19BrCl2N2O7 CAS NO.87531-10-0 MW610.24, Assay 98%, Storage: 2-8°C
-5-(5-((E)-2-bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-(((4-chlorobenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-chlorobenzoate.png)
DMT-dT Phosphoramidite, 5′-O-(4,4′-dimethoxytrityl)-2′-deoxythymidine-3′-O-[O-(2-cyanoethyl)-N,N′-diisopropylphosphoramidite], 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-deoxythymidine, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], DMT-dT amidite, Thymidine, 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], C40H49N4O8P CAS NO.98796-51-1 MW744.81, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.74%, Storage: -20°C, protect from light, stored under nitrogen, [1]. James D Thorpe, et al. Mechanochemical Synthesis of Short DNA Fragments. Chemistry. 2020 Jul 22;26(41):8857-8861.

3'-O-DMTr-thymidine 5'-CE phosphoramidite, C40H49N4O8P CAS NO.134031-86-0 MW744.81, Assay 98%, Storage: 2-8°C

DMT-2'-F-dA Phosphoramidite, C40H47FN7O6P CAS NO.252770-65-3 MW771.82, Assay 98%,
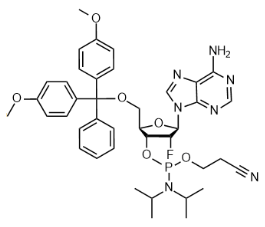
5'-O-DMT-2'-O-TBDMS-N-Bz-Adenosine, C44H49N5O7Si CAS NO.81265-93-2 MW787.97, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 96.75%, Storage: 2-8°C, protect from light, It is a compound containing fluorescent adenosine, which is commonly used to detect DNA/RNA modifications or add synthetic oligonucleotide blocks
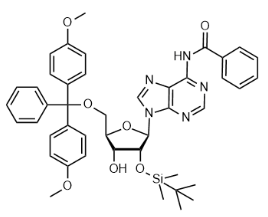
5'-DMT-3'-TBDMS-Bz-rA, N-(9-((2R,3R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-((tert-butyldimethylsilyl)oxy)-3-hydroxytetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide, C44H49N5O7Si CAS NO.81256-88-4 MW787.97, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥97.0%, Storage: 2-8°C, protect from light, It is used for nucleoside modification, amidite and nucleic acid synthesis.

DMT-dC(bz) Phosphoramidite, Cytidine, N-benzoyl-5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-deoxy-, 3′-[2-cyanoethyl bis(1-methylethyl)phosphoramidite], DMT-dC(bz) amidite, N-benzoyl-5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-deoxycytidine, 3′-[2-cyanoethyl N,N-?bis(1-methylethyl)phosphoramidite], N4-Benzoyl-5′-O-(4,4′-dimethoxytrityl)-2′-deoxycytidine-3′-O-[O-(2-cyanoethyl)-N,N′-diisopropylphosphoramidite], N4-Benzoyl-2’-deoxy-5’-O-DMT-cytidine 3’-CE Phosphoramidite, C46H52N5O8P CAS NO.102212-98-6 MW833.91, form SOlid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.70%, mp>84 °C,Storage: 2-8°C, protect from light, stored under nitrogen, DMT-dC(bz) phosphoramidite is a DNA phosphoramidite. Its main characteristics include: the amino group outside the ring is protected by benzoyl (dA(bz) and dC(bz)) or isobutyryl (dG(ib)). For oligonucleotides protected with standard bases, the recommended conditions for deprotection are 55°C for 8 hours or room temperature for 24 hours. The high coupling efficiency of DNA phosphoramidites by Proligo results in high yields and high quality oligonucleotides. [1]. James D Thorpe, et al. Mechanochemical Synthesis of Short DNA Fragments. Chemistry. 2020 Jul 22;26(41):8857-8861. [2]. Prakash, T., et al.: J. Med. Chem., 53, 1636 (2010); Winkler, J., et al.: Eur. J. Med. Chem., 44, 670 (2009)
 Phosphoramidite.png)
N-(9-((2R,3R,4R,5R)-5-((Bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-3,4-bis((tert-butyldimethylsilyl)oxy)tetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide, N-(9-((2R,3R,4R,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-3,4-bis((tert-butyldimethylsilyl)oxy)tetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide(WXC08861), C50H63N5O7Si2 CAS NO.160513-05-3 MW902.24, Assay 95%
-5-((Bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-3,4-bis((tert-butyldimethylsilyl)oxy)tetrahydrofuran-2-yl)-9H-purin-6-yl)benzamide.png)
ATP (disodium salt hydrate), Adenosine 5'-triphosphate (disodium salt hydrate), C10H14N5O13Na2P3.xH2O CAS NO.34369-07-8 MW551.14 (anhydrous basis), form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥99.0%, mp176°C, Storage: 2-8°C, sealed storage, away from moisture
.png)
Uridine 5'-diphosphate (sodium salt), C9H14N2O12P2.xNa CAS NO.21931-53-3 MW404.16 (free acid basis), form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation: -6.42°(C=0.91g/100mI, H2O, 20°C, 589nm), solubility H2O: soluble 100 mg/mL, clear, colorless to very faintly yellow, Storage: -20°C, stored under nitrogen, Application UDP has been used as a purinergic agonist to study its effects on absorptive cationic flux in cochlear outer sulcus cells (OSC) and vestibular transitional cells (VTC). UDP has also been used for nucleoside diphosphatase (NDPase) activity assays in rabbit retinae. Biochem/physiol Actions P2Y receptor agonist. Preparation Note Uridine 5′-(trihydrogen diphosphate) sodium or UDP sodium salt is soluble in water at 100 mg/ml and yields a clear, colorless to very faint yellow solution. [1]. J H Lee, T Chiba, D C Marcus, P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. The Journal of neuroscience : the official journal of the Society for Neuroscience (2001-11-22) [2].J Schnitzer, Enzyme-histochemical demonstration of microglial cells in the adult and postnatal rabbit retina. The Journal of comparative neurology (1989-04-08)
.png)
Adenosine 5'-diphosphate (sodium salt), C10H15N5O10P2.xNa CAS NO.20398-34-9 MW427.20+ X22.99, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, bp877.7°C at 760 mmHg, Flash point 484.6°C, Solubility: Aqueous Base (Slightly), DMSO (Slightly), Methanol(Slightly), Water (Slightly), Storage: -20°C, sealed storage, away from moisture and light, [1]. Adenosine-5'-diphosphate [2]. Arts IC, et al. Adenosine 5'-triphosphate (ATP) supplements are not orally bioavailable: a randomized, placebo-controlled cross-over trial in healthy humans. J Int Soc Sports Nutr. 2012 Apr 17;9(1):16. [3]. Noel J. Cusack, et al. Effects of phosphate-modified analogs of adenosine 5′-diphosphate and adenosine 5′‐triphosphate at P2T-purinoceptors mediating human platelet activation by ADP. April 1996. [4]. David Erlinge, et al. P2Y receptors in health and disease. Adv Pharmacol. 2011:61:417-39. [5]. David Iyú, et al. Adenosine derived from ADP can contribute to inhibition of platelet aggregation in the presence of a P2Y12 antagonist. Arterioscler Thromb Vasc Biol. 2011 Feb;31(2):416-22.
.png)
meso-Erythritol, C4H10O4 CAS NO.149-32-6 MW122.12, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp117-121°C, bp330°C at 760 mmHg, Solubility: Methanol (Slightly), Water (Soluble), Storage: -20°C, stored under nitrogen, [1]. Hootman KC, et al. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc Natl Acad Sci U S A. 2017 May 23;114(21):E4233-E4240. [2]. ángel E Mercado-Pagán, et al. Hemocompatibility evaluation of small elastomeric hollow fiber membranes as vascular substitutes. J Biomater Appl. 2014 Oct;29(4):557-65. [3]. Arun Sreekumar, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [4]. Cinzia Formighieri, et al. Carbon partitioning to the terpenoid biosynthetic pathway enables heterologous β-phellandrene production in Escherichia coli cultures. Arch Microbiol. 2014 Dec;196(12):853-61. [5]. Edward Sisco, et al. Rapid detection of sugar alcohol precursors and corresponding nitrate ester explosives using direct analysis in real time mass spectrometry. Analyst. 2015 Apr 21;140(8):2785-96. [6]. Leisso, R., et al.: J. Agr. Food. Chem., 61, 1373 (2013); Hillmann, H., et al.: J. Agr. Food. Chem., 60, 9974 (2012);
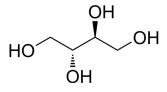
Thyminose, Deoxyribose, 2-Deoxy-D-ribose, C5H10O4 CAS NO.533-67-5 MW134.13, Solubility: Aqueous Acid (Slightly,Sonicated), DMSO (Slightly),Methanol (Slightly), form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=1.0 g/100ml H2O): -57.2°, Residue on Ignition: 0.10%, mp89-90°C, bp379.7°C at 760 mmHg, Storage: 2-8°C, stored under nitrogen, 2-Deoxy-D-ribose is used as a precursor to deoxyribonucleic acid. It induces apoptosis by inhibiting the synthesis of glutathione and increasing the efflux of glutathione. Also, it is used in the synthesis of optically active dipyrrhamnol from the surface of montmorillon KSF clay. [1]. Dong Gu Lee, et al. Novel Dammarane-Type Triterpene Saponins from Panax ginseng Root. Chem Pharm Bull (Tokyo). 2015;63(11):927-34. [2]. Ganiyu Oboh, et al. Essential Oil from Clove Bud (Eugenia aromatica Kuntze) Inhibit Key Enzymes Relevant to the Management of Type-2 Diabetes and Some Pro-oxidant Induced Lipid Peroxidation in Rats Pancreas in vitro. J Oleo Sci. 2015;64(7):775-82. [3]. J B Bollyky, et al. Evaluation of in vivo T cell kinetics: use of heavy isotope labelling in type 1 diabetes. Clin Exp Immunol. 2013 Jun;172(3):363-74. [4]. Jinping Jia, et al. Comparison of Fruits of Forsythia suspensa at Two Different Maturation Stages by NMR-Based Metabolomics. Molecules. 2015 May 29;20(6):10065-81. [5]. Md Mahbubur Rahman, et al. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015 Oct 30:8:621. [6]. Fico, A., et al.: Free Radical Biology & Medicine, 45, 211 (2008),

L-Xylose, L-(-)-Xylose, C5H10O5 CAS NO.609-06-3 MW150.13, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR[α](C=1.0g/100ml H2O): -46.3°, Water(KF): 0.16%, Residue on Ignition: 0.02%, mp150-152 °C, bp191.65 °C at 760 mmHg, solubility: DMSO (Slightly), Water (Slightly), Storage: Storage temp. 2-8°C, Building Blocks; Carbohydrates and Oligosaccharides; L-Xylose is used in the synthesis of L-Xylose derivatives as selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the treatment of type 2 diabetes. [1]. Wang XX, et al. The implementation of high fermentative 2,3-butanediol production from xylose by simultaneous additions of yeast extract, Na2EDTA, and acetic acid. N Biotechnol. 2015 Aug 3. [2]. Goodwin, N.C., et. al.: J. Med. Chem., 52, 6201 (2009)
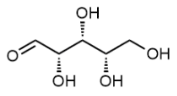
L-Ribose, L-(+)-Ribose, C5H10O5 CAS NO.24259-59-4 MW150.13, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, mp81-82°C, bp415.5°C at 760 mmHg, Solubility: Methanol (Slightly), Water(Slightly), Storage: -20°C, stored under nitrogen, It is produced by microorganism fermentation of glucose in a fermentation culture medium without adding calcium carbonate. L-Ribose is a potential starting material for the synthesis of many L-nucleoside-based drug compounds. [1]. Jiyoung A Hong, et al. Identification of critical ligand binding determinants in Mycobacterium tuberculosis adenosine-5'-phosphosulfate reductase. J Med Chem. 2009 Sep 10;52(17):5485-95. [2]. Rocky Strollo, et al. Antibodies to post-translationally modified insulin in type 1 diabetes. Diabetologia. 2015 Dec;58(12):2851-60. [3]. Sachio Yamamoto, et al. Chiral separation of D/L-aldoses by micellar electrokinetic chromatography using a chiral derivatization reagent and a phenylboronic acid complex. Anal Bioanal Chem. 2015 Aug;407(20):6201-6. [4]. Sirinan Shompoosang, et al. Enzymatic production of three 6-deoxy-aldohexoses from L-rhamnose. Biosci Biotechnol Biochem. 2014;78(2):317-25. [5]. Ostrowski, S., et al.: Science, 305, 71 (2004), Vavilin, D., et al.: Biochim. Biophys. Acta, 1708, 91

D-Ribose(mixture of isomers), C5H10O5 CAS NO.50-69-1 MW150.13, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=0.7060,H2O): -18.6°, mp95°C, bp375.4°C at 760 mmHg, Solubility: Methanol (Slightly), Water (Sparingly), Storage: Storage temp. 2-8°C, D-Ribose is produced by microorganism fermentation of glucose in a fermentation culture medium without adding calcium carbonate. [1]. Hong J, et al. D-ribose induces nephropathy through RAGE-dependent NF-κB inflammation. Arch Pharm Res. 2018 Aug;41(8):838-847. [2]. W E Wick, et al. Compound 64716, a new synthetic antibacterial agent. Antimicrob Agents Chemother. 1973 Oct;4(4):415-20. [3]. Angelika Kaczyńska, et al. Sensitization of HER2 Positive Breast Cancer Cells to Lapatinib Using Plants-Derived Isothiocyanates. Nutr Cancer. 2015;67(6):976-86. [4]. Eric A Lee, et al. Targeting Mitochondria with Avocatin B Induces Selective Leukemia Cell Death. Cancer Res. 2015 Jun 15;75(12):2478-88. [5]. Jiyoung A Hong, et al. Identification of critical ligand binding determinants in Mycobacterium tuberculosis adenosine-5'-phosphosulfate reductase. J Med Chem. 2009 Sep 10;52(17):5485-95. [6]. Ostrowski, S., et al.: Science, 305, 71 (2004), Vavilin, D., et al.: Biochim. Biophys. Acta, 1708, 91
.png)
L-(+)-Arabinose C5H10O5 CAS NO.5328-37-0 MW150.13, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥99.0%, Optical Rotation[a]: 111.136°(C=0.01 g/ml H2O ), Water(KF): 0.51%, mp160-163 °C, bp415.5±38.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, L-Arabinose is a naturally occurring isomer that is a component of plant polysaccharides. Most bacteria contain an inducible arabin operon that encodes a series of enzymes and transporters that allow L-arabinose to be used as the sole carbon source in a microbial culture. [1]. Huck JH, et al. Ribose-5-phosphate isomerase deficiency: new inborn error in the pentose phosphate pathway associated with a slowly progressive leukoencephalopathy. Am J Hum Genet. 2004 Apr;74(4):745-51. [2]. Osaki S, et al. L-arabinose feeding prevents increases due to dietary sucrose in lipogenic enzymes and triacylglycerol levels in rats. J Nutr. 2001 Mar;131(3):796-9. [3]. Agne Tubeleviciute, et al. Escherichia coli kduD encodes an oxidoreductase that converts both sugar and steroid substrates. Appl Microbiol Biotechnol. 2014 Jun;98(12):5471-85. [4]. Ahyun Son, et al. M1 RNA is important for the in-cell solubility of its cognate C5 protein: Implications for RNA-mediated protein folding. RNA Biol. 2015;12(11):1198-208. [5]. David P Ward, et al. Centrifugal partition chromatography in a biorefinery context: Separation of monosaccharides from hydrolysed sugar beet pulp. J Chromatogr A. 2015 Sep 11:1411:84-91.
-Arabinose.png)
D-Arabitol, C5H12O5 CAS NO.488-82-4 MW152.15, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Solubility: Aqueous Sodium Borate (Slightly), DMSO (Slightly), Methanol (Slightly), mp101-104 °C, bp494.5°C at 760 mmHg, Storage: Storage temp. 2-8°C, Carbohydrates and Oligosaccharides; D-Arabinitol, can be used as a diagnostic tool for the detection of fungal infections in neutropenic patients. It can be used as a sugar for the production of xylitol through the process of biotransformation. It is a substrate used for the identification, distinction, characterization of enzymes, such as oxidizing glucose dehydrogenase from Zymomonas mobilis, Gox2181, hyperthermophilic Darabitol dehydrogenase from marine Thermotoga, and NAD-dependent D-arabitol dehydrogenase from acetic acid bacteria, Alkalithermomon fusca. [1]. Klusmann A, et al. Influence of D-arabitol and ribitol on neuronal network activity. J Inherit Metab Dis. 2005;28(6):1181-3. [2]. Astrid R Mach-Aigner, et al. L-arabitol is the actual inducer of xylanase expression in Hypocrea jecorina (Trichoderma reesei). Appl Environ Microbiol. 2011 Sep;77(17):5988-94. [3]. Meizhong Luo, et al. A geographical discrimination of Shanxi extra aged vinegars using polyalcohols as the discriminators. J AOAC Int. 2013 Sep-Oct;96(5):1048-53. [4]. Mingguo Jiang, et al. Microbiological purification of L-arabitol from xylitol mother liquor. J Microbiol Biotechnol. 2011 Jan;21(1):43-9. [5]. Srujana Koganti, et al. Production of arabitol from glycerol: strain screening and study of factors affecting production yield. Appl Microbiol Biotechnol. 2011 Apr;90(1):257-67 [6]. Salonen, J.H., et al.: Eur. J. Clin. Microbiol. Infect. Dis., 20, 179 (2001);

Methyl-β-D-xylopyranoside, Methyl-beta-D-xylopyranoside, C6H12O5 CAS NO.612-05-5 MW164.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Purity (NMR): ≥98.0%, mp155-158 °C, bp314.0±42.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. D J Carey, et al. Effects of inhibition of proteoglycan synthesis on the differentiation of cultured rat Schwann cells. J Cell Biol. 1987 Aug;105(2):1013-21. [2]. Mária Mastihubová, et al. Deoxy and deoxyfluoro analogues of acetylated methyl beta-D-xylopyranoside--substrates for acetylxylan esterases. Carbohydr Res. 2004 Aug 23;339(12):2101-10. [3]. P B Gordon, et al. Extracellular matrix proteoglycans and cell-substratum adhesion of human endothelial cells: the effect of methyl beta-D-xylopyranoside. Carbohydr Res. 1986 Aug 15:151:121-34. [4]. Peter Biely, et al. Mode of action of acetylxylan esterase from Streptomyces lividans: a study with deoxy and deoxy-fluoro analogues of acetylated methyl beta-D-xylopyranoside. Biochim Biophys Acta. 2003 Jul 23;1622(2):82-8. [5]. Peter Biely, et al. Transacetylations to carbohydrates catalyzed by acetylxylan esterase in the presence of organic solvent. Biochim Biophys Acta. 2003 Oct 13;1623(2-3):62-71.
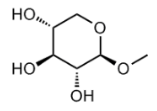
Rhamnose, L-Rhamnose, 6-deoxy-L-mannose, C6H12O5 CAS NO.3615-41-6 MW164.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp91-95°C, bp399.1°C at 760 mmHg, Storage: Storage temp. 2-8°C
(-)-Fucose, 6-Desoxygalactose, L-(-)-Fucose, L-Galactomethylose, 6-Deoxy-L-galactose, L-Galactomethylose, C6H12O5 CAS NO.2438-80-4 MW164.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.55%, mp150-153°C, bp323.9°C at 760 mmHg, Solubility: Methanol (Slightly, Heated), Water (Slightly), Storage: -20°C, stored under nitrogen, L-Fucose (6-Deoxy-L-galactose) is used in the study of alginatecontaining polysaccharides. It is investigated as a polysaccharide modifier of carbohydrates that can generate antigenic sites recognized by IgE antibodies. L-Fucose used as a substrate to identify, distinguish, and characterize enzymes such as alginate lyase, L-fucose isomerase, and L-fucose degenase. It can be used in the study of organelles and bacterial microcompartments involved in the degradation of sugars in plant and algal cell walls. L-ucose can also be used as a reference compound for the identification and analysis of rare sugars. [1]. Becker DJ, et al. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003 Jul;13(7):41R-53R. [2]. Artur Rogowski, et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun. 2015 Jun 26:6:7481. [3]. Chen Chen, et al. Affinity ligands for glycoprotein purification based on the multi-component Ugi reaction. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Oct 15:969:171-80. [4]. Hai-ming Chen, et al. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015 Feb 1:168:302-10. [5]. Hua Tian, et al. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int J Biol Macromol. 2015 Aug:79:577-82.
-Fucose.png)
Methyl α-D-xylopyranoside,Methyl alpha-D-xylopyranoside,C6H12O5 CAS NO.91-09-8 MW164.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Storage temp. 2-8°C
L-Fucitol, 1-Deoxy-D-galactitol, C6H14O5 CAS NO.13074-06-1 MW166.17, form Solid, color Off-white to light yellow, Assay (HPLC): 99.13%, mp154-156 °C, bp468.0±40.0 °C at 760 mmHg, Solubility: Methanol (Slightly), Water (Slightly), Storage: 2-8°C, protect from light, L-Fucitol is a sugar alcohol that is isolated from nutmeg. [1]. Farag, M. A. , et al. Sensory metabolites profiling in Myristica fragrans (Nutmeg) organs and in response to roasting as analyzed via chemometric tools. LWT, 2018,97, 684–692.

L-Gulono-1,4-lactone, L-Gulonolactone, C6H10O CAS NO.1128-23-0 MW178.14, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp187-190°C, bp467.9°C at 760 mmHg, Solubility:DMSO (Slightly), Water(Slightly), Storage: Storage temp. 2-8°C, L-Gulono-1,4-lactone is a precursor in the biosynthesis of ascorbic acid, one of the many forms of Vitamin C. [1]. Wolucka BA, et al. Mycobacterium tuberculosis possesses a functional enzyme for the synthesis of vitamin C, L-gulono-1,4-lactone dehydrogenase. FEBS J. 2006 Oct;273(19):4435-45. Epub 2006 Sep 5. [2]. Wolucka, B. et al.: J. Biol. Chem., 278, 47483 (2003); Heller, R. et al.: J. Biol Chem., 274, 8254 (1999);
D-Glucono-1,4-lactone, C6H10O6 CAS NO.1198-69-2 MW178.14, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, mp134-135 °C, bp467.9±18.0 °C at 760 mmHg, Solubility:DMSO (Slightly), Methanol(Slightly), Water (Slightly), Storage: Storage temp. 2-8°C, Applications: D-Glucaric acid-1,4-lactone is an organic compound of carboxylic acid ester, which can be used as pharmaceutical. D-Glucono-1,4-lactone was a metabolite studied in mice tissue with induced chronic stress. [1]. Hamiliton, P. J., et al.: Sci. Rep., 10, 18134 (2020)
L-Glucose, L-(-)-Glucose, C6H12O6 CAS NO.921-60-8 MW180.16, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=1.00 g/100ml, H2O): -57.8°, mp153-156 °C, bp232.96 °C at 760 mmHg, Storage: Storage temp. 2-8°C, L-(-)-glucose is the enantiomer of the more common D-glucose, a natural carbohydrate used in large quantities of cellular. L-glucose is a synthetic sugar used to form L-glucose pentacetate, a potential therapeutic agent for type II diabetes. In addition, L-glucose can be used as a colon cleanser for colonoscopy. [1]. Wang QP, et al. Chronic Sucralose or L-Glucose Ingestion Does Not Suppress Food Intake. Cell Metab. 2017 Aug 1;26(2):279-280. [2]. Claudia St?ubert, et al. Hydroxycarboxylic acid receptors are essential for breast cancer cells to control their lipid/fatty acid metabolism. Oncotarget. 2015 Aug 14;6(23):19706-20. [3]. Fan Li, et al. Structure and bioactivity of a polysaccharide extracted from protocorm-like bodies of Dendrobium huoshanense. Int J Biol Macromol. 2015 Jan:72:664-72. [4]. Inkie J A Evers-van Gogh, et al. Electric Pulse Stimulation of Myotubes as an In Vitro Exercise Model: Cell-Mediated and Non-Cell-Mediated Effects. Sci Rep. 2015 Jun 19:5:10944. [5]. Jose C Paz, et al. Combinatorial regulation of a signal-dependent activator by phosphorylation and acetylation. Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17116-21. [6]. Malaisse, W. et al.: Int. J. Mol. Med., 2, 383 (1998); Raymer, G. et al.: Gastrointest. Endosc., 58, 30 (2003); Wang, R., et al.: J. Mol. Catal. B. Enz., 56, 131 (2009),
D-Tagatose, D-(-)-Tagatose, C6H12O6 CAS NO.87-81-0 MW180.16, form Solid, color White to off-white, Assay: ≥98.0%, mp130-136 °C, bp232.96 °C at 760 mmHg, Solubility:DMSO (Slightly), Water(Slightly), Storage: Storage temp. 2-8°C, A monosaccharide (hexose) that can be used as a low-calorie sweetener, as an intermediate for synthesis of other optically active compounds, and as an additive in detergent, cosmetic, and pharmaceutical formulation. [1]. Jeong DW, et al. Trienzymatic Complex System for Isomerization of Agar-Derived d-Galactose into d-Tagatose as a Low-Calorie Sweetener. J Agric Food Chem. 2020;68(10):3195-3202. [2]. Daniel J Wichelecki, et al. ATP-binding Cassette (ABC) Transport System Solute-binding Protein-guided Identification of Novel d-Altritol and Galactitol Catabolic Pathways in Agrobacterium tumefaciens C58. J Biol Chem. 2015 Nov 27;290(48):28963-76. [3]. Edwige Van der Heiden, et al. Synthesis and Physicochemical Characterization of D-Tagatose-1-Phosphate: The Substrate of the Tagatose-1-Phosphate Kinase in the Phosphotransferase System-Mediated D-Tagatose Catabolic Pathway of Bacillus licheniformis. J Mol Microbiol Biotechnol. 2015;25(2-3):106-19. [4]. Moez Rhimi, et al. Production of D-tagatose, a low caloric sweetener during milk fermentation using L-arabinose isomerase. Bioresour Technol. 2011 Feb;102(3):3309-15. [5]. Moez Rhimi, et al. The acid-tolerant L-arabinose isomerase from the mesophilic Shewanella sp. ANA-3 is highly active at low temperatures. Microb Cell Fact. 2011 Nov 10:10:96. [6]. Oh, D. et al.: Appl. Microbiol. Biotech., 76, 76, 1 (2007); Lu, Y. et al.: Int. J. Cosm. Sci., 24, 225 (2002); Donner, T.W. et al.: Diab. Obes. Metab., 1, 285 (1999);

D-Glucose, D-(+)-Glucose, C6H12O6 CAS NO.50-99-7 MW180.156, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥96.0%, Optical Rotation[a]: 96.9789°(C=0.5594g/100ml H2O), Residue on Ignition: 0.07%, mp150-152°C, bp527.1°C at 760 mmHg, Solubility:DMSO (Slightly), Methanol(Slightly), Water (Sparingly), Storage: Storage temp. 2-8°C, [1]. Jin Jiaojiao, et al. D-glucose, D-galactose, and D-lactose non-enzyme quantitative and qualitative analysis method based on Cu foam electrode. Food Chem. 2015 May 15;175:485-93. [2]. Ying Liu, et al. Podocyte-Released Migrasomes in Urine Serve as an Indicator for Early Podocyte Injury. Kidney Dis (Basel). 2020 Nov;6(6):422-433. [3]. Andy Maloney, et al. Effect of (2)H and (18)O water isotopes in kinesin-1 gliding assay. PeerJ. 2014 Mar 25:2:e284. [4]. Anja T?uber, et al. Comparison of the antifungal efficacy of terbinafine hydrochloride and ciclopirox olamine containing formulations against the dermatophyte Trichophyton rubrum in an infected nail plate model. Mol Pharm. 2014 Jul 7;11(7):1991-6. [5]. Cong-Fei Xu, et al. Targeting glucose uptake with siRNA-based nanomedicine for cancer therapy. Biomaterials. 2015 May:51:1-11. [6]. Wang, R., et al.: J. Mol. Catal. B. Enz., 56, 131 (2009); Springhorn, C. et al.: J. Clin. Endocrinol. Metab., 97, 4640 (2012); Hashimoto, K. et al.: J. Clin. Endocrinol. Metab., 97, 3016 (2012); Avelange, M. et al.: Plant. Phys., 94, 1157 (1990); Cramer, C. et al.: J. Chem. Soc., 115, 5745 (1993);
2,3-O-Isopropylidene-D-ribonolactone, C8H12O5 CAS NO.30725-00-9 MW188.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): 98.0%, mp135-138 °C, bp243.17 °C at 760 mmHg, Storage: Storage temp. 2-8°C, 2,3-O-Isopropylidene-D-ribonic acid-1,4-lactone is used the synthesis of C-nucleosides, prostaglandins, and GABA analogs via pure chiral 2-piperidone. It serves as a starting for the preparation of biochemical compounds including C-nucleosides (such as neplanocin A) and GABA analogs via pure chiral 2-piperone.

1,2-O-Isopropylidene-alpha-D-xylofuranose,Monoacetone-D-xylose, C8H14O5 CAS NO.20031-21-4 MW190.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR[α](C=0.678g/100ml H2O): -18.5°, Water(KF): 0.03%, mp69-71°C, bp333.0±37.0 °C at 760 mmHg, Flash point 155.2±26.5 °C, Storage: Storage temp. 2-8°C, 1,2-O-Isopropylidene-alpha-D-xylofuranose is an intermediate used in the synthesis of C-glycosyl nucleosides and nucleoside aldehydes. [1]. Zhang, P., et al.: Youji Huaxue, 36, 1690 (206)
D-Glucuronic acid, C6H10O7 CAS NO.6556-12-3 MW194.14, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=0.12g/100ml H2O): 19.6°, mp159-161°C, bp398.1°C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Teng F, et al. Production of d-glucuronic acid from myo-inositol using Escherichia coli whole-cell biocatalyst overexpressing a novel myo-inositol oxygenase from Thermothelomyces thermophile. Enzyme Microb Technol. 2019 Aug;127:70-74. [2]. Amanda K Chaplin, et al. GlxA is a new structural member of the radical copper oxidase family and is required for glycan deposition at hyphal tips and morphogenesis of Streptomyces lividans. Biochem J. 2015 Aug 1;469(3):433-44. [3]. Artur Rogowski, et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun. 2015 Jun 26:6:7481. [4]. Daoyuan Ren, et al. Antioxidant and antitumor effects of polysaccharides from the fungus Pleurotus abalonus. Chem Biol Interact. 2015 Jul 25:237:166-74. [5]. Jie Zhang, et al. Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities. Phytochemistry. 2014 Dec:108:157-70.
Methyl α-D-galactopyranoside, C7H14O6 CAS NO.3396-99-4 MW194.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (GC): 99.84%, Optical Rotation: 175.3°(C=1.02g/100mL, H2O, 20°C, 589nm), mp116-117 °C, bp250.62 °C at 760 mmHg, Storage: Storage temp. 2-8°C, Methyl α-D-galactopyranoside serves as an effective inhibitor against extracellular and intracellular α-galosidase of Hansenula polymorpha UFV-1. [1]. Alexander I Zinin, et al. 1-O-Acetyl-beta-D-galactopyranose: a novel substrate for the transglycosylation reaction catalyzed by the beta-galactosidase from Penicillium sp. Carbohydr Res. 2002 Apr 2;337(7):635-42. [2]. Andrew J Nok, et al. A 45-kDa midgut glycoprotein from Anopheles albimanus mosquito mediates the killing of trypanosomes. Cell Biochem Funct. 2002 Sep;20(3):257-62. [3]. Ann-Sofie Lepp?nen, et al. Metal-mediated allylation of enzymatically oxidized methyl α-D-galactopyranoside. Carbohydr Res. 2010 Dec 10;345(18):2610-5. [4]. Atta-ur-Rahman, et al. alpha-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus. J Nat Prod. 2008 May;71(5):910-3. [5]. H Takesada, et al. The interaction of elderberry (Sambucus sieboldiana) bark lectin and sialyloligosaccharides as detected by 1H-NMR. J Biochem. 1992 Jul;112(1):143-6.

D(+)-Galactosamine (hydrochloride), D-Galactosamine HCl, C6H14ClNO5 CAS NO.1772-03-8 MW215.63, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 97.52%, mp182-185 °C, bp449°C at 760 mmHg, Solubility: Methanol (Slightly, Heated),Water (Slightly), Storage: 2-8°C, sealed storage, away from moisture, For biochemical research. The substrate used to measure the activity of aromatic amine N-methyltransferase and monoamine oxidase in tissue homogenate. Specifically inhibits COX-2 by preventing COX-2 N-glycosylation and by increasing COX-2 protein turnover in a proteasome-dependent manner. [1]. Ayhanci A, et al. Protective effects of ellagic acid in D-galactosamine-induced kidney damage in rats. Cytotechnology. 2016;68(5):1763-1770. [2]. Okumura A, et al. Alleviation of lipopolysaccharide/d-galactosamine-induced liver injury in leukocyte cell-derived chemotaxin 2 deficient mice. Biochem Biophys Rep. 2017;12:166-171. Published 2017 Oct 13. [3]. C Hubert, et al. Towards a full integration of optimization and validation phases: An analytical-quality-by-design approach. J Chromatogr A. 2015 May 22:1395:88-98. [4]. E I Lafferty, et al. An ENU-induced splicing mutation reveals a role for Unc93b1 in early immune cell activation following influenza A H1N1 infection. Genes Immun. 2014 Jul-Aug;15(5):320-32. [5]. Inoue, H., et al.: J. Biol. Chem., 270, 24965
-Galactosamine (hydrochloride).png)
D-Mannosamine (hydrochloride), (2S,3R,4S,5R)-2-Amino-3,4,5,6-tetrahydroxyhexanal (hydrochloride), C6H14ClNO5 CAS NO.5505-63-5 MW215.63, form Solid, color White to off-white (Solid)1 H NMR Spectrum: Consistent with structurePurity (NMR): ≥98.0%, mp168°C, bp532.5°C at 760 mmHg, Solubility:Methanol (Very Slightly,Heated, Sonicated), Water(Slightly, Sonicated), Storage: 2-8°C, stored under nitrogen, D-Mannosamine hydrochloride is used in the synthesis of non-natural Mannac analogs for expressing thiols the cell surface sialic acids. It is also used in the study and screening of a library of N-acetyl-β-D-glucosaminid inhibitors against osteoarthritis. It is a precursor to N-acetylneuraminic acid and other N-acyl precursors of sialic. It is a drug compound used in the development of mannosylated liposomes for bioadhesive oral drug delivery through Peyer's patch M cells. [1]. Christopher Brigham, et al. Sialic acid (N-acetyl neuraminic acid) utilization by Bacteroides fragilis requires a novel N-acetyl mannosamine epimerase. J Bacteriol. 2009 Jun;191(11):3629-38. [2]. Cristina Y Zamora, et al. Sialidases as regulators of bioengineered cellular surfaces. Glycobiology. 2015 Jul;25(7):784-91. [3]. Edward Bodnar, et al. Mass spectrometric analysis of products of metabolic glycan engineering with azido-modification of sialic acids. Anal Bioanal Chem. 2015 Dec;407(30):8945-58. [4]. Filippo Bonaccorsi, et al. A new route for the synthesis of Streptococcus pneumoniae 19F and 19A capsular polysaccharide fragments avoiding the beta-mannosamine glycosylation step. Carbohydr Res. 2009 Aug 17;344(12):1442-8. [5]. Peter Chefalo, et al. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine. Biochemistry. 2006 Mar 21;45(11):3733-9. [6]. Pukanud, P. et al.: Drug Delivery, 16, 289 (2009); Knowles, D.J. et al.: J. Gen. Microbiol., 108, 17 (1978);
.png)
Glucosamine (hydrochloride), D-(+)-Glucosamine hydrochloride, Chitosamine hydrochloride, C6H14ClNO5 CAS NO.66-84-2 MW215.63, form Solid, colof White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp190-194°C, Solubility: Methanol (Slightly, Heated,Sonicated), Water (Sparingly), Storage: 2-8°C, sealed storage, away from moisture, Novel application of glucosamine to prepare medical agent for treating vertigo. Found in chitin, in mucoproteins, and in mucopolysaccharides. Antiarthritic. Recent studies show that its chondroprotective activity is related to its antiapoptic properties. [1]. Zupanets, I.A. et al.: Klin. Farmat., 15, 67 (2011); Vajarudal, Y., et al.: Clin. Ther., 3, 336 (1981); Setnikar, I., et al.: Arzneim.-Forsch., 36, 729 (1986);
.png)
D-Glucurono-6,3-lactone acetonide, 1,2-O-Isopropylidene-Alpha-D-glucofuranosidurono-6,3-lactone, C9H12O6 CAS NO.20513-98-8 MW216.19, form Solid, color White to off-white, Assay (GC): 98.68%, mp119-121°C, bp386.0±42.0 °C at 760 mmHg, Storage: Store at room temperature, (3aR,3bS,6S,6aR,7aR)-6-Hydroxy-2,2-dimethylthydrofuro[2',3':4,5]furo[2,3-d][1,3]dioxol-5(3aH )- (20513-98-8) is a compound that can be used in organic synthesis.

Gluconate (sodium), D-Gluconic acid sodium salt, Sodium D-gluconate, D-Gluconate sodium salt, C6H11NaO7 CAS NO.527-07-1 MW218.14, mp170-175°C, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.01%, Solubility: Methanol (Slightly), Water(Slightly), Storage: 2-8°C, sealed storage, away from moisture, Sodium D-Gluconate has use as an additive in processed foods, with the potential to improve taste. It has also been shown through study to alter gene expression in the colon of colorectal cancer model subjects. This is the labeled isomer. [1]. Narukawa, M. et al.: Biosci. Biotech. Biochem., 76, 2282 (2012); Kameue, C. et al.: Biosci. Biotech. Biochem., 70, 606 (2006);
.png)
D-N-Acetylgalactosamine, C8H15NO6 CAS NO.1811-31-0 MW221.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥97.0%, mp158-162°C, bp636.4°C at 760 mmHg, Storage: Storage temp. 2-8°C, N-Acetyl-D-galactosamine is a bio-activated reporters to visualize. N-acetyl-D-galactosamine is the initial O-linked sugar on many serine and threonine residues in protein glycosation. [1]. Antonina Gucciardi, et al. A column-switching HPLC-MS/MS method for mucopolysaccharidosis type I analysis in a multiplex assay for the simultaneous newborn screening of six lysosomal storage disorders. Biomed Chromatogr. 2014 Aug;28(8):1131-9. [2]. Antonino Tuttolomondo, et al. A family with various symptomatology suggestive of Anderson-Fabry disease and a genetic polymorphism of alpha galactosidase A gene. Clin Biochem. 2015 Jan;48(1-2):55-62. [3]. Haruko Ogawa, et al. Autoactivation of pancreatic trypsinogen is controlled by carbohydrate-specific interaction. FEBS Lett. 2015 Feb 27;589(5):569-75. [4]. M Z Lei, et al. Tumor necrosis factor-like weak inducer of apoptosis regulates the phenotype and cytotoxic activity of goat uterine natural killer cells. J Anim Sci. 2015 Feb;93(2):589-97. [5]. Mark E Diebel, et al. Estrogen modulates intestinal mucus physiochemical properties and protects against oxidant injury. J Trauma Acute Care Surg. 2015 Jan;78(1):94-9. [6]. Nakajima, M., et al.: App. Microbiol. Biotechnol., 83, 109 (2009), Singh, S., et al.: Mol. Immunol., 46, 1240 (2009),

Cyclic N-Acetyl-mannosamine, C8H15NO6 CAS NO.1136-44-3 MW221.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.19%, bp595.4±50.0 °C at 760 mmHg, Storage: Storage temp. -20°C

N-Acetyl-D-glucosamine, N-Acetyl-2-amino-2-deoxy-D-glucose, C8H15NO6 CAS NO.7512-17-6 MW221.21, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp211°C, bp636.4°C at 760 mmHg, Storage: -20°C, stored under nitrogen, N-Acetyl-D-glucosamine (GlcNAc) oligomers may have the ability to elicit canine polymorphonuclear leukocyte (PMN) factors in vivo. It has wound healing and chemotactic activity. GlcNAc and its derivatives are generally used in the preparation of dietary supplements and are also used in drug development. [1]. Slawson C, et al. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006 Jan 1;97(1):71-83. [2]. A Rizzo, et al. Melanoma cells homing to the brain: an in vitro model. Biomed Res Int. 2015:2015:476069. [3]. Carolina Fontana, et al. Structural studies and biosynthetic aspects of the O-antigen polysaccharide from Escherichia coli O42. Carbohydr Res. 2015 Feb 11:403:174-81. [4]. Hui Xiong, et al. Triterpene saponins with anti-inflammatory activity from the stems of Entada phaseoloides. Fitoterapia. 2015 Jun:103:33-45. [5]. J Richter, et al. Collagen-induced arthritis: severity and immune response attenuation using multivalent N-acetyl glucosamine. Clin Exp Immunol. 2014 Jul;177(1):121-33.
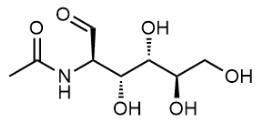
D-Gluconic acid (potassium), D-Gluconic Acid Monopotassium Salt, Gluconic acid potassium salt, Gluconsan K, Helshas K, K-IAO, Kalimozan, Kalium-Beta, Kaon, Katorin, Katrin, Potalium, Potasoral, Potassium D-gluconate, Potassium gluconate, Potassuril, Sirokal, Tumil K, C6H11KO7 CAS NO.299-27-4 MW234.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp183 °C, Solubility: Methanol (Slightly), Water(Slightly), Storage: Store at room temperature, keep dry and cool, Potassium D-gluconate is the potassium salt of gluconic acid and is used in cell culture and large-scale applications, such as cleaning surfaces and fertilizers. D-Gluconic Acid is used as a reagent in the synthesis of Pangamic Acid (P179500, Ca Salt); a mineral supplement that is sometimes referred to as Vitamin B15 although it is not a true vitamin. [1]. Kaur R, et al. Gluconic acid: an antifungal agent produced by Pseudomonas species in biological control of take-all. Phytochemistry. 2006 Mar;67(6):595-604. [2]. CEDEF, et al. [Item 232--Facial dermatoses: acne]. Ann Dermatol Venereol. 2012 Oct;139(11 Suppl):A192-6. [3]. Hongwei Yu, et al. CaMKII Controls Whether Touch Is Painful. J Neurosci. 2015 Oct 21;35(42):14086-102. [4]. Luca Rolle, et al. CIEL*a*b* parameters of white dehydrated grapes as quality markers according to chemical composition, volatile profile and mechanical properties. Anal Chim Acta. 2012 Jun 30:732:105-13. [5]. Masataka Narukawa, et al. Evaluation of the suppressive effect on bitter taste of gluconate. Biosci Biotechnol Biochem. 2012;76(12):2282-8. [6]. Mansurova, I.D. et al.: Eks. Patol. Pech., 1, 58 (1973); Apanasenko, A.A.: Cor Vasa, 15, 20 (1973); Alkhanova, N.A.: Nauch. Tru. Kaz. Gosud. Med. Inst., 41, 83 (1974)
.png)
(2S,3R,4R,5R)-5-Methyltetrahydrofuran-2,3,4-triyl triacetate, 1,2,3-Triacetyl-5-deoxy-β-D-Riboturanose, C11H16O7 CAS NO.62211-93-2 MW260.24, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp63-64°C, bp315.3°C at 760 mmHg, Flash point 136°C, Solubility: Chloroform (Slightly), Dichloromethane (Sparingly), Methanol (Sparingly, Sonicated), Storage: Storage temp. 2-8°C, Intermediate of Capecitabine, [1]. Wang, G., et al.: J. Med. Chem., 43, 2566 (2000),
-5-Methyltetrahydrofuran-2,3,4-triyl triacetate.png)
1,2:3,4-Di-O-isopropylidene-α-D-galactopyranose, C12H20O6 CAS NO.4064-06-6 MW260.28, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: Storage temp. 2-8°C, Diacetyl-D-galactose is used to produce diacetyl-d-galactose aldehyde and oxalic acid, It is mainly used in biochemical reactions and pharmaceutical intermediates.
Diacetone-D-mannitol, D-Mannitol 1,2:5,6-bis-acetonide, 1,2:5,6-Di-O-isopropylidene-D-mannitol, C12H22O6 CAS NO.1707-77-3 MW262.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.10%, mp120-122°C, bp382°C at 760 mmHg, Solubility: Chloroform (Slightly), Dichloromethane, Methanol (Slightly), Storage: Storage temp. 2-8°C, Nebivolol intermediate. [1]. Ono, Y., et al.: Steroids, 71, 529 (2006),

Tri-O-Acetyl-D-galactal, D-Galactal Triacetate, C12H16O7 CAS NO.4098-06-0 MW272.25, form , color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.32%, mp28 - 30°C, bp138-140 °C at 760 mmHg, Solubility: Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, 3,4,6-Tri-O-acetyl-D-galactal is an important component in oligosacchar solutions and solid-phase synthesis.

Tri-O-acetyl-D-glucal, C12H16O7 CAS NO.2873-29-2 MW272.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.99%, Water(KF): 0.07%, OR[α](C=1.20 g/100ml ETOH): -14.4°, mp53-55°C, bp343.1°C at 760 mmHg, Flash point >113°C, Solubility: Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, 3,4,6-Tri-O-acetyl-D-glucosealdoxime is used as a component in solution and solid-phase oligosaccharide synthesis, furthermore, it is also used in the preparation of D-arabino-1,5-anhydro-2-de-hex-1-enitol.

Arbutin, β-Arbutin, C12H16O7 CAS NO.497-76-7 MW272.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.76%, mp198-201°C, bp561.6°C at 760 mmHg, Flash point 293.4°C, Solubility: DMSO (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, Arbutin is a glycosylated hydroquinone extracted from bearberry plant. Arbutin is a known inhibitor of tyrosinase, which in turn prevents the formation of melanin. Arbutin is often used as a skin-lightening agent in cosmetic products. [1]. Magnusson, B., et al.: J. Invest. Dermatol., 52, 268 (1969), Goodwin, B., et al.: Contact Dermatitis, 7, 248, (1981), ,Itoh, M., et al.: J. Dermatol., 9, 223 (1982), Sugimoto, K., et al.: Biol. Pharm. Bull., 24, 510 (2004)
Methyl 4,6-O-Benzylidene-α-D-glucopyranoside, Methyl 4,6-O-Benzylidene-alpha-D-glucopyranoside, C14H18O6 CAS NO.3162-96-7 MW282.29, form Solid, color White to off-white, Assay: ≥95.0%, mp164-167 °C, bp473 °C at 760 mmHg, Solubility: Chloroform, DMF, DMSO, Ethyl Acetate, Methanol, Storage: Storage temp. 2-8°C, Methyl 4,6-O-Benzylidene-a-D-glucopyranoside is a derivative of methyl a-D-glucopyranoside tested for in vitro antibacterial activity. [1]. Namazi H, et, al. Regioselective synthesis of vinylic derivatives of common monosccarides through their activated stannylene acetal intermediates. Molecules. 2005 Aug 31;10(7):772-82. [2]. Andary, C, et al.: Phytochem., 21, 1123 (1982),

n-Octyl β-D-glucopyranoside, C14H28O6 CAS NO.29836-26-8 MW292.37, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=1.00g/100ml MEOH): -32.1°, Water(KF): 0.33%, mp103-104 °C, bp354.3 °C at 760 mmHg, Solubility: DMSO (Slightly), Water(Slightly), Storage: Storage temp. 2-8°C, n-Octyl-β-D-glucoside (βOG) assists in the extraction, dissolution, and denaturation of membrane proteins, Octyl glucoside has diverse applications including: reconstitution of ion channels, dissolution of enzymes, and protein incorporation into liposomes. Additionally, nonionic detergent are also used in protein crystallography studies. It is primarily used in the expression and purification of recombinant proteins. [1]. Gould RJ, et al. Effects of octyl beta-glucoside on insulin binding to solubilized membrane receptors. Biochemistry. 1981 Nov 24;20(24):6776-81. [2]. Konidala P, et al. Molecular dynamics characterization of n-octyl-beta-D-glucopyranoside micelle structure in aqueous solution. J Mol Graph Model. 2006 Sep;25(1):77-86. [3]. Joana A Loureiro, et al. Charged surfactants induce a non-fibrillar aggregation pathway of amyloid-beta peptide. J Pept Sci. 2013 Sep;19(9):581-7. [4]. Jürgen Borlak, et al. Proteome mapping of epidermal growth factor induced hepatocellular carcinomas identifies novel cell metabolism targets and mitogen activated protein kinase signalling events. BMC Genomics. 2015 Feb 25;16(1):124. [5]. Luke L Lairson, et al. Using substrate engineering to harness enzymatic promiscuity and expand biological catalysis. Nat Chem Biol. 2006 Dec;2(12):724-8. [6]. Lairson, L., et al.: Nat. Chem. Biol., 2, 724 (2006),
4-Nitrophenyl α-D-glucopyranoside, C12H15NO8 CAS NO.3767-28-0 MW301.25,form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp210-216 °C, bp582.2 °C at 760 mmHg, Solubility: Ethanol (Slightly, Heated), Methanol (Slightly), Water(Slightly, Heated, Sonica), Storage: Storage temp. 2-8°C, 4-Nitrophenyl-α-D-glucopyranoside is used as a substrate for the Glycosidase. After hydrolysis, the substrate is converted into a yellow product. p-Nitrophenyl a-D-Glucopyranoside is a substrate for a-glucosidase inhibitor. [1]. Binder TP, et, al. p-Nitrophenyl alpha-D-glucopyranoside, a new substrate for glucansucrases. Carbohydr Res. 1983 Dec 23;124(2):287-99. [2]. Zeng L, et, al. Inhibitory mechanism of morin on α-glucosidase and its anti-glycation properties. Food Funct. 2016 Sep 14;7(9):3953-63. [3]. P S Unnikrishnan, et al. Alpha-amylase Inhibition and Antioxidant Activity of Marine Green Algae and its Possible Role in Diabetes Management. Pharmacogn Mag. 2015 Oct;11(Suppl 4):S511-5. [4]. Suphongphan Srisurichan, et al. Pregnane-type steroidal glycosides from Gymnema griffithii Craib. Phytochemistry. 2014 Oct:106:197-206. [5]. O'Neill, R., et al.: J. Biol. Chem., 264, 20430
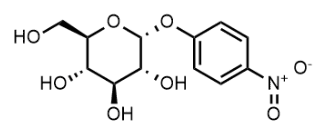
((3aR,5R,6R,6aR)-6-Hydroxy-2,2,6-trimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)methyl benzoate, C16H20O6 CAS NO.16831-81-5 MW308.33, Assay 98%, mp110-111 °C, bp430.5±40.0 °C at 760 mmHg
-6-Hydroxy-2,2,6-trimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)methyl benzoate.png)
N-Acetylneuraminic Acid, 5-(Acetylamino)-3,5-dideoxy-D-glycero-Dgalacto-2-nonulosonic Acid, C11H19NO9 CAS NO.131-48-6 MW309.27, form Solid, color White to Off-White, Assay 96%(HPLC), Solubility: Methanol (Slightly, Heated), Water (Slightly), Storage: Storage temp. -20°C, N-Acetyl-neuraminic Acid is a useful reagent in sequential esterification and acetylation of Sialyl phosphite and phosphoramidite. [1]. Methods Enzymol., 5, 391 (1962), Biochemistry, 18, 2086 (1979)

Phenylmethyl 2-(acetylamino)-2-deoxy-α-D-glucopyranoside, C15H21NO6 CAS NO.13343-62-9 MW311.33, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp183-185°C, bp594.9±50.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-2-deoxy-α-D-glucopyranoside.png)
1,2,3,5-Tetra-O-acetyl-beta-L-ribofuranose, [(2S,3S,4S,5R)-3,4,5-triacetyloxyoxolan-2-yl]methyl acetate, C13H18O9 CAS NO.144490-03-9 MW318.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Solubility: Chloroform (Sparingly), Methanol (Slightly), Pyridine (Slightly), Storage: 2-8°C, sealed storage, away from moisture, 1,2,3,5-Tetra-O-acetyl β-L-Ribofuranose is an isomer of 1,2,3,5-Tetra-O-acetyl b-D-Ribofuranose (T283100) which is used in the synthesis of 3-(β-D-ribofuranosyl)-2,3-dihydro-6H-1,3-oxazine-2,6-dione, a new pyrimidine nucleoside analog related to uridine. [1]. Chwang, T.-L., et al.: J. Med. Chem., 19, 643 (1976)

Esculin, C15H16O9 CAS NO.531-75-9 MW340.28, form Solid, color White to Off-White,NMR Conforms to Structure,MS Conforms to Structure,Assay (HPLC): 98.21% (340 nm), Specific Rotation: -54.7° (c = 0.1, DMSO), Water: ~8.1% based on Elemental Analysis, Solubility: DMSO (Slightly), Methanol(Slightly), Storage: 4°C, Esculin is a coumarin glucoside occuring in horse chestnut. It is used in the identification of bacterial species such as listeria strains. [1]. Adzitey, F. et al.: J. Biol. Sci., 13, 614 (2013); Achyut Bharadwaj, S. et al.: J. Chem. Pharm., 4, 119 (2011);

1,2-Di-O-acetyl-5-O-benzoyl-3-deoxy-3-fluoro-D-ribofuranose, C16H17FO7 CAS NO.159099-24-8 MW340.30, form Oil, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, bp422.9±45.0 °C at 760 mmHg, Storage: Storage temp. -20°C

4-Nitrophenyl-N-acetyl-β-D-glucosaminide, C14H18N2O8 CAS NO.3459-18-5 MW342.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 99.51%, mp210-212 °C, bp477.77 °C at 760 mmHg, Storage: Storage temp. 2-8°C, 4-Nitrophenyl-N-acetyl-β-D-glucosamine (NP-GlcNAc) is a used substrate for hexosaminidases. The enzymatic action cleaves the glycosidic bond and forms 4-nitrophenol (pNP), being measured at 405 nm. NP-GlcNAc can also be used in combination with diethylaminoethyl-α-cyclodextrin (DE-CD) for the rapid and accurate determination of the rate of β-N-acetylglucosaminidase. The cyclodextrin derivative is used as additive, which ionizes 4-nitrophenol into yellow 4-nitrophenolate at a pH value close to 5 (the optimum pH value of the enzyme).

Maltitol, C12H24O11 CAS NO.585-88-6 MW344.31, form Solid, color White to Off-White Solid White, NMR Conforms to Structure, Elemental Analysis Conforms %C: 41.86, %H: 7.32, MS Conforms to Structure, HPLC Assay: 96.79% (ELSD), Solubility: Methanol (Slightly), Water (Soluble), Storage: -20°C, Maltitol is a sugar alcohol, used as a sugar replacer. [1]. Livesey, Geoffrey., et al.: Nutrition Research Reviews, 16,163 (2003);
2,3,4,6-Tetra-O-acetyl-D-glucopyranose, C14H20O10 CAS NO.10343-06-3 MW348.30, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp137-138 °C, bp425.0±45.0 °C at 760 mmHg, Solubility: Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly), Water (Slightly), Storage: Storage temp. 2-8°C, 2,3,4,6-Tetra-O-acetyl-D-glucopyranose is a compound useful in organic synthesis.

D-Lactose Monohydrate, C₁₂H₂₄O₁₂, CAS NO.64044-51-5 MW360.31, form Solid, color White to Off-White, Elemental Analysis Conforms %C: 40.02, %H: 6.88, NMR Conforms to Structure, MS Conforms to Structure, HPLC Assay 100.00% (ELSD), Specific Rotation: +48.8° (c = 1.0, Water; 4 Hours), Water Content 4.9% by Karl Fischer, Solubility: DMSO (Sparingly), Water(Sparingly), Storage: 4°C, D-Lactose Monohydrate is an excipient used in dry powder inhaler of aspirin. [1]. Bais, N., et al.: Int. J. Pharm. Life Sci., 7, 5047-5050 (2016)

(2R,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl dodecanoate, C18H34O7 CAS NO.64395-91-1 MW362.46, Assay 98%, mp128-133 °C, Storage: 4°C,
-3,4,5-Trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl dodecanoate.png)
1-Thio-beta-D-glucose tetraacetate, C14H20O9S CAS NO.19879-84-6 MW364.37, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp115-117 °C, bp425.5±45.0 °C at 760 mmHg, Solubility: Chloroform (Slightly), Methanol (Slightly), Storage: 2-8°C, stored under nitrogen, 1-Thio-beta-D-glucose tetraacetate is used as an inhibitor in the Maillard reaction between glucose and glycine

Chloro 2-acetamido-2-deoxy-3,4,6-tri-O-acetyl-α-D-glucopyranose, 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-glucopyranosyl Chloride, C14H20ClNO8 CAS NO.3068-34-6 MW365.76, form Solid, color White to off-white (Solid), 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥97.0%, mp>214°C, bp516.4±50.0 °C at 760 mmHg, Storage: -20°C, sealed storage, away from moisture
2-Deoxy-2,2-difluoro-D-erythro-pentonic acid γ-Lactone 3,5-dibenzoate, ((2R,3R)-3-(Benzoyloxy)-4,4-difluoro-5-oxotetrahydrofuran-2-yl)methyl benzoate, C19H14F2O6 CAS NO.122111-01-7 MW376.31, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=0.751 CHCL3): 47.4°, mp117-119°C, bp437.2°C at 760 mmHg, Storage: Store at room temperature, 2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate is a compound useful in organic synthesis.
Methyl 2-deoxy-3,5-di-O-toluoyl-D-ribofuranoside, C22H24O6 CAS NO.4330-34-1 MW384.42, form Liquid, color Light brown to brown Assay (LCMS): 95.80%, Solubility: Chloroform (Slightly), Ethyl Acetate (Slightly), Storage: Storage temp. 2-8°C, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.

2-Deoxy-3,5-di-O-p-toluoyl-D-erythro-pentofuranosyl chloride, C21H21ClO5 CAS NO.3601-89-6 MW388.84, form Solid, color White to off-white, Purity (HPLC): 96.98%, mp109°C, bp518.4°C at 760 mmHg, Flash point 185.6°C, Solubility: Acetonitrile (Slightly), Chloroform (Slightly), Dichloromethane (Slightly), DMSO (Slightly), Storage: Storage temp. 2-8°C, A general carbohydrate derivative, widely used in the preparation of 2'-deoxyribonucleosides.
(2R,3S,5R)-5-Chloro-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-methylbenzoate, C21H21ClO5 CAS NO.4330-21-6 MW388.84, form Solid, color Off-white to gray, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥90.0%, mp117-119 °C, bp518.4°C at 760 mmHg, Storage: Storage temp. -20°C
-5-Chloro-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-methylbenzoate.png)
β-D-Galactosamine pentaacetate, 2-Acetamido-1,3,4,6-tetra-O-acetyl-2-deoxy-β-D-galactopyranose, (2S,3R,4R,5R,6R)-3-Acetamido-6-(acetoxymethyl)tetrahydro-2H-pyran-2,4,5-triyl Triacetate, C16H23NO10 CAS NO.3006-60-8 MW389.35, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.04%, OR(C=0.732g/100ml CHCL3): 3.4°, mp234-236°C, bp530.2±50.0 °C at 760 mmHg, Solubility: Chloroform (Slightly, Heated,Sonicated), DMSO (Slightly, Heated), Methanol (Slightly), Storage: Storage temp. 2-8°C, (2S,3R,4R,5R,6R)-3-Acetamido-6-(acetoxymethyl)tetrahydro-2H-pyran-2,4,5-triyl Triacetate is used to synthesize selenium monosaccharide as metabolic biomarker. [1]. Deng, Shengyuan. et al., Faming Zhuanli Shenqing(2020);
D-Glucose pentaacetate, Penta-O-acetyl-D-glucopyranose, C16H22O11 CAS NO.83-87-4 MW390.34, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, mp130-132°C, bp452 °C at 760 mmHg, Storage: Storage temp. 2-8°C

Acetobromo-α-D-glucuronic acid methyl ester, Acetobromo-alpha-D-glucuronic acid methyl ester, (2R,3R,4S,5S,6S)-2-Bromo-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate, C13H17BrO9 CAS NO.21085-72-3 MW397.17, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, Solubility: Acetonitrile (Slightly), Chloroform (Slightly, Heated,Sonicated), Dichloromethane (Slightly)Storage: -20°C, stored under nitrogen, Acetobromo-a-D-glucuronic Acid Methyl Ester is a useful compound for preparing a fluorogenic probe for human heparanase. [1]. Honda, T., et al.: J. Med. Chem., 31, 1295 (1988), [2]. Liu, J., et al.: Chem. Sci., (2020)
(2R,3R,4S,5R,6S)-2-(Acetoxymethyl)-6-(acetylthio)tetrahydro-2H-pyran-3,4,5-triyl triacetate, 2,3,4,6-tetra-O-acetyl-1-S-acetyl-1-thiohexopyranose, C16H22O10S CAS NO.13639-50-4 MW406.40, form Solid, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Store at room temperature
-2-(Acetoxymethyl)-6-(acetylthio)tetrahydro-2H-pyran-3,4,5-triyl triacetate.png)
5-Bromo-4-chloro-3-indolyl b-D-Galactopyranoside, X-GAL, C14H15BrClNO6 CAS NO.7240-90-6 MW408.63, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.86%, mp230°C, Solubility: DMF (Slightly), Methanol (Slightly), Storage: 2-8°C, protect from light, A substrate for b-Galactosidase that is converted by the enzyme into an intense indigo-blue chromophore (615 nm). [1]. Horwitz, J. et al.: J. Med. Chem. 1964 Jul;7:574-5.Lojda, Z. et al.: Histochemie 1973 Mar 26;34(4):361-9.

Dapagliflozin, (1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol, (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, CAS NO.461432-26-8, form Solid, color White to Pale Yellow, NMR Conforms to Structure, MS Conforms to Structure, HPLC Assay 99.86% (200 nm), Solubility: DMSO (Slightly), Methanol (Slightly), Storage: -20°C, A sodium-glucose transporter 2 inhibitor. [1]. Kasichayanula, S. et al.: Diabetes, Obesity and Metabolism 13, 47 (2011)
2,3,4,6-Tetra-O-acetyl-α-D-glucopyranosyl Bromide (stabilized with 1% CaCO3), α-Acetobromoglucose, contains 1% CaCO3 as stabilizer, Bromo 2,3,4,6-Tetra-O-acetyl-Alpha-D-glucopyranoside (~ 5% CaCO3 as stabilizer), C14H19BrO9 CAS NO.572-09-8 MW411.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Optical Rotation[a]: 177.4°(C=0.01g/ml CHCL3), Water(KF): 0.5%, mp84-89 °C, bp412.0±45.0 °C at 760 mmHg, Solubility: Acetontrile (Very Slightly), Chloroform (Slightly), Methanol (Slightly), Storage: -20°C, stored under nitrogen, Bromo 2,3,4,6-Tetra-O-acetyl-a-D-glucopyranoside (1~ 5% CaCO3 as stabilizer) is useful for preparing kauranes for the treatment of antibiotic-resistant bacterial infections. [1]. Imperi, F., et al.: PCT Int. Appl. (2021), WO 2021014422 A1
.png)
Liquiritin, 4′,7-Dihydroxyflavanone 4′-glucoside, Liquiritoside, (S)-2-[4-(β-D-Glucopyranosyloxy)phenyl]-2,3-dihydro-7-hydroxy-4H-1-benzopyran-4-one, 4',7-Dihydroxyflavanone 4'-β-Dglucopyranoside, 4',7-Dihydroxyflavanone 4'-β-D-Glucoside, 7-Hydroxyflavanone 4'-OGlucoside, Likviritin, Liquiritigenin 4'-β-Dglucopyranoside, C21H22O9 CAS NO.551-15-5 MW418.39, form Solid, color White to Off-White, NMR Conforms to Structure, HPLC Assay 98.86% (220 nm), MS Conforms to Structure, Solubility: DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly), Liquiritin is a major constituent of Glycyrrhiza Radix that has a neuroprotective effect against glutamate toxicity in DPC12 cells and may have potential for the treatment of neurodegenerative diseases. [1]. Teng, L., et. al.: Mol. Med. Reports, 10, 818 (2014)

2,3,5-Tri-O-benzyl-D-ribono-1,4-lactone, C26H26O5 CAS NO.55094-52-5 MW418.49, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (LCMS): 97.30%(205nm), mp54-55°C, bp576.8±50.0 °C at 760 mmHg, Solubility: Chloroform (Sparingly), DMSO (Slightly, Sonicated), Methanol (Slightly), Storage: Storage temp. 2-8°C, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.

(2R,3R,4R,5S,6R)-2-phenethoxy-6-((((3R,4R,5R)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methoxy)methyl)tetrahydro-2H-pyran-3,4,5-triol, C20H30O10 MW430.45, Assay 98%
-2-phenethoxy-6-((((3R,4R,5R)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methoxy)methyl)tetrahydro-2H-pyran-3,4,5-triol.png)
Methyl 1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucopyranoside, (3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol, C22H27ClO7 CAS NO.461432-24-6 MW438.90, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 96.50%, Storage: 2-8°C, stored under nitrogen, away from moisture, DAG-5A (CAS# 461432-24-6) is an intermediate of Dapagliflozin.
methyl]phenyl]-D-glucopyranoside.png)
Baicalin, Baicalein 7-O-β-D-glucuronide, C21H18O11 CAS NO.21967-41-9 MW446.36, form Solid, color Light yellow to yellow, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 95.23%, mp202-205°C, bp836.6°C at 760 mmHg, Solubility: DMSO (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, Baicalin, Baicalin is the glucuronide of Baicalein. Baicalin is a known prolyl endopeptidase inhibitor, induces apoptosis in pancreatic cancer cells,[3] and affects the GABA receptors. [1]. Tarrago, T., et al.: Bioorg. Med. Chem, 16 (15), 7516 (2008); [2]. Takahashi, H., et al.: Biochim Biophys Acta, 1813 (8), 1465 (2011);
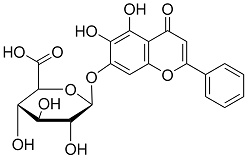
MAC glucuronide linker-2, (2S,3R,4S,5S,6S)-2-(2-amino-4-(hydroxymethyl)phenoxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate, C20H25NO11 CAS NO.229977-57-5 MW455.41, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.38%, bp572.5±50.0 °C at 760 mmHg, Storage: 2-8°C, stored under nitrogen, [1]. Robert A, et al. Compounds, compositions, and methods for treatment of diseases involving acidic or hypoxic diseased tissues. WO2019136298A1.
(3R,4S,5S,6R)-6-(hydroxymethyl)-2-methoxy-2-(4-methyl-3-((5-phenylthiophen-2-yl)methyl)phenyl)tetrahydro-2H-pyran-3,4,5-triol, C25H28O6S MW456.56, form Solid, Assay 95%, Storage: Store at room temperature
-6-(hydroxymethyl)-2-methoxy-2-(4-methyl-3-((5-phenylthiophen-2-yl)methyl)phenyl)tetrahydro-2H-pyran-3,4,5-triol.png)
Hyperoside, 2-(3,4-Dihydroxyphenyl)-3-(β-Dgalactopyranosyloxy)-5,7-dihydroxy-4H-1- benzopyran-4-one, 3,3',4',5,7- Pentahydroxyflavone 3-O-β-Dgalactopyranoside, Quercetin 3-O-β-Dgalactoside, C21H20O12, CAS NO.482-36-0 MW464.38, form Solid, color Light Yellow to Yellow, NMR Conforms to Structure, MS Conforms to Structure, Elemental Analysis Conforms %C: 52.26, %H: 4.44, HPLC Assay 99.05% (255 nm), Specific Rotation Report Result -2.0° (c = 0.1, Methanol), Solubility: DMSO (Slightly), Methanol (Sparingly), Water (Slightly), Storage: -20°C, A major flavonoid in apple peels; a bioactive constituent of apple peels with potent antioxidant and antiproliferative activities. [1]. Willett, W., et al.: Science, 264, 532

2-Deoxy-2-fluoro-α-D-arabinofuranose 1,3,5-tribenzoate, C26H21FO7 CAS NO.97614-43-2 MW464.44, form SOlid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.42%, OR(C=0.719g/100ml CDCL3): 76.7°, Water(KF): 0.06%, mp74-77°C, bp584.1°C at 760 mmHg, Solubility: Chloroform (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, Clofarabine intermediate. [1]. Williams, A., et al.: Biochem., 48, 11994 (2009), Pathak, A., et al.: Bioorg. Med. Chem., 17, 872 (2009),

(3R,4S,5R,6R)-3,4,5-Tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one, 2,3,4,6-Tetrakis-O-trimethylsilyl-D-gluconolactone, d-2,3,4,6-tetrakis-o-(trimethylsilyl)-gluconic acid delta-lactone, C18H42O6Si4 CAS NO.32384-65-9 MW466.36, bp417.2±45.0°C at 760 mmHg, Flash point 171.3±24.3°C, Solubility: Chloroform (Sparingly), Ethyl Acetate (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (GC): 99.10%, Optical Rotation: 46.568°(C=0.01g/ml CHCL3), 2,3,4,6-Tetrakis-O-trimethylsilyl-D-gluconolactone is an antidiabetic agent and an intermediate of Dapagliflozin (D185370). 2,3,4,6-Tetrakis-O-trimethylsilyl-D-gluconolactone acts as a reagent in the synthesis of trans-cyclohexane-bearing C-glucose as sodium glucose co-transporter 2 inhibitors. [1]. Shao, H., et al.: Youji Huaxue, 31, 836 (2011);
-3,4,5-Tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one.png)
Methyl 1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucopyranoside, C25H27FO6S CAS NO.1030825-21-8 MW474.54, bp644.5±55.0 °C at 760 mmHg, (2R,S)-2-Methoxy Canagliflozin is an impurity of Canagliflozin (CAT# C175190), which is a sodium-glucose cotransporter-2 inhibitor (SGLT2i) useful in the treatment of type 2 diabetes and proven to ameliorate kidney and heart failure in patients with type 2 diabetes. It can be useful in the synthesis and characterization of potential Impurities of Canagliflozin. [1]. Minami, T., et al.: Expert Opin. Pharmacother., 22, 2087 (2021); [2]. Wada, T., et al.: J. Diabetes Invest., 13, 54 (2022); [3]. Sen, T., et al.: J. Am. Heart Assoc., 10, e021661 (2021); [4]. Pachore, S. S., et al.: ChemistrySelect, 2, 9157 (2017)
-2-thienyl]methyl]-4-methylphenyl]-D-glucopyranoside.png)
(3R,4S,5S,6R)-2-(3-((5-(2-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol, C25H27FO6S MW474.55, Assay (NMR) 98%, Storage: Store at room temperature
-2-(3-((5-(2-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol.png)
1,3,4,6-Tetra-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranose, C22H23NO11 CAS NO.10022-13-6 MW477.42, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.51%, mp200 °C, bp577.3±50.0 °C at 760 mmHg, Solubility: Acetone, Chloroform, Dichloromethane, Ethyl Acetate, Storage: Storage temp. 2-8°C, 2-Deoxy-2-N-phthalimido-1,3,4,6-tetra-O-acetyl-b-D-glucopyranose (cas# 10022-13-6) is a compound useful in organic synthesis. [1]. Zhang, G., et al.: Bioorg. Med. Chem., 11, 3273 (2003),
1-O-Acetyl-2,3,5-Tri-O-benzoyl-beta-D-Ribofuranose, C28H24O9 CAS NO.6974-32-9 MW504.48, form Solid, color White to off-white, Assay 98%, mp128-130°C, bp621°C at 760 mmHg, 2-8°C, Solubility: Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly, Sonicated), Storage: sealed storage, away from moisture, 1-O-Acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose (6974-32-9) is an inhibitor of neutrophil-lactoferrin adhesion. Anti-inflammatory. Azacitidine USP related compound B. [1]. Shappell, S., et al.: J. Immunol., 144, 2702

Picroside II,C23H28O13 CAS NO.39012-20-9 MW512.46, form SOlid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.61%, mp145-155°C, Solubility: DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly), Storage: 2-8°C, protect from light, Picroside II similar to Picroside I, acts as an oxygen free radical scavenger and thus promotes anti-inflammatory activity and may be used in the treatment of arthritis or ischemic injury. [1]. Wang, Y. et al.: Res. Neuro. Int. J., 488911, 8 (2014); [2]. Singh, G. et al.: Phytother. Res., 7, 402 (1993); [3]. Chander R. et al.: Biochem. Pharmacol., 7, 190 (1992);
(2S,3R,4S,5S)-5-((R)-1-hydroxy-2-(pivaloyloxy)ethyl)tetrahydrofuran-2,3,4-triyl tris(2,2-dimethylpropanoate), β-D-Galactofuranose 1,2,3,6-Tetrakis(2,2-diMethylpropanoate), C26H44O10 CAS NO.226877-02-7 MW516.62, Assay 95%, bp563.4±50.0 °C at 760 mmHg, Storage: 2-8°C
-5-((R)-1-hydroxy-2-(pivaloyloxy)ethyl)tetrahydrofuran-2,3,4-triyl tris(2,2-dimethylpropanoate).png)
(2S,3R,4S,5S)-5-((S)-1-hydroxy-2-(pivaloyloxy)ethyl)tetrahydrofuran-2,3,4-triyl tris(2,2-dimethylpropanoate), α-L-Altrofuranose, 1,2,3,6-tetrakis(2,2-dimethylpropanoate), C26H44O10 CAS NO.226877-04-9 MW516.62, Assay 95%, bp563.4±50.0 °C at 760 mmHg, Storage: 2-8°C
-5-((S)-1-hydroxy-2-(pivaloyloxy)ethyl)tetrahydrofuran-2,3,4-triyl tris(2,2-dimethylpropanoate).png)
Melezitose Monohydrate, C18H32O16.H2O CAS NO.10030-67-8 MW522.45236, form SOlid, color White to Off-White, Assay >95% (HPLC), Solubility: Methanol, Water (Slightly), Storage: -20℃, Melezitose Monohydrate is an energy rich triacylglycerol used in potential biofuels development. [1]. Holder, J. et al.: PLoS One, 7, e1002219 (2011);

Oleuropein, (2S,3E,4S)-3-Ethylidene-2-(β-D-glucopyranosyloxy)-3,4-dihydro-5-(methoxycarbonyl)-2H-pyran-4-acetic acid 2-(3,4-dihydroxyphenyl)ethyl ester, C25H32O13 CAS NO.32619-42-4 MW540.51, form Solid, color Off-White to Pale Brown, White to Faint Yellow and Faint Beige and Faint Brown, NMR Conforms to Structure, Elemental Analysis Conforms %C: 54.43, %H: 5.92, MS Conforms to Structure, Specific Rotation Report Result -182.1° (c = 0.1, Ethanol), HPLC Assay 96.38% (205 nm), Solubility: DMSO (Slightly), Ethanol (Slightly, Sonicated), Methanol (Slightly), Storage: 4°C, application(s) metabolomics vitamins, nutraceuticals, and natural products, Oleuropein is a tyrosol ester derivative of elenolic acid and phenolic glycoside found in olive oil. Oleuropein shows antioxidant, anti-ischemic and hypolipidemic activity. Oleuropein has also been used as an immunomodulator. [1]. Speroni, E. et al.: Phytother. Res., 12, S98 (1998); [2]. Andreadou, I. et al.: J. Nutrit., 136, 2213 (2006); [3]. Giamarellos-Bourboulis, E.J. et al.: Shock, 26, 410 (2006);
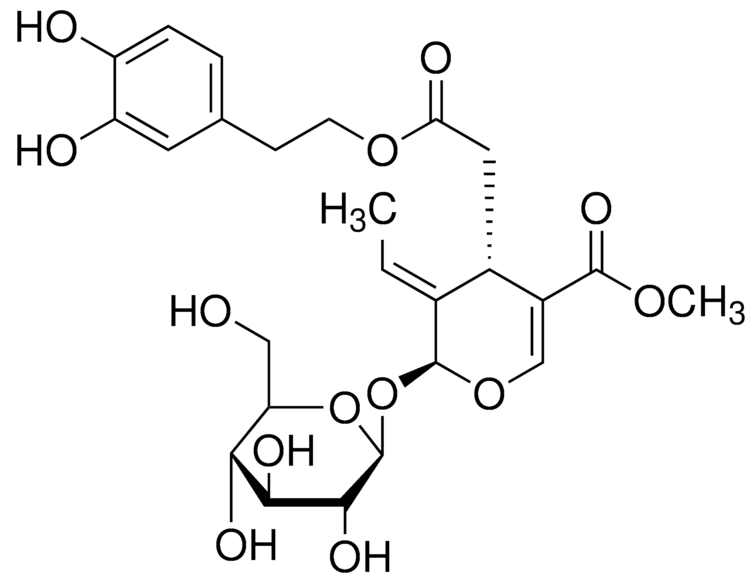
2,3,4,6-Tetra-O-benzyl-alpha-D-glucopyranose, 2,3,4,6-Tetrakis-O-(phenylmethyl)-α-D-glucopyranose, C34H36O6 CAS NO.6564-72-3 MW540.65, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (HPLC): 98.43%, mp151-151°C, bp672.4±55.0°C at 760 mmHg, Storage: Storage temp. 2-8°C, Intermediate of voglibose/dapagliflozin. An important D-glucopyranoside derivative, used for glucosyl and other reactions 1,2; preparation of α-glucopyranosyl chloride, synthesis of 1-C-α-D-glucopyranide derivatives.
2,3,4,6-Tetra-O-benzyl-D-glucopyranose, 2,3,4,6-Tetrakis-O-(phenylmethyl)-D-glucopyranose, C34H36O6 CAS NO.4132-28-9 MW540.66, form Solid, color White to off-white, NMR Conforms to Structure, MS Conforms to Structure, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 99.17%(205nm), Water(KF): 0.21%, mp145-149°C, bp672.4°C at 760 mmHg, Solubility: Chloroform (Slightly), Dioxane (Sparingly), Methanol (Slightly), Storage: Storage temp. 2-8°C, 2,3,4,6-Tetra-O-benzyl-D-glucopyranose (cas# 4132-28-9) is a compound useful in organic synthesis.
Hexadecyl β-D-maltoside, hexadecyl 4-O-alpha-D-glucopyranosyl-beta-D-glucopyranoside, C28H54O11 CAS NO.98064-96-1 MW566.72, form Solid, Assay 98%, Storage: 2-8°C, Hexadecyl β-D-maltopyranoside used as a detergent for the purification, extraction, and solubilization of membrane-bound proteins, [1]. Fu F, et al. Surface Properties of Alkyldi(oxyethylene) β-D-Maltoside. J Agric Food Chem. 2022 Mar 2;70(8):2643-2655.
(1S)-1,5-Anhydro-1-C-[3-(benzo[b]thien-2-ylMethyl)-4-fluorophenyl]-D-glucitol 2,3,4,6-tetraacetate, C29H29FO9S CAS NO.1034305-21-9 MW572.60, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, bp634.8±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-1,5-Anhydro-1-C-[3-(benzo[b]thien-2-ylMethyl)-4-fluorophenyl]-D-glucitol 2,3,4,6-tetraacetate.png)
D-(+)-Raffinose Pentahydrate, C18H32O16.5H2O CAS NO.17629-30-0 MW594.51, NMR Conforms to structure, MS Conforms to Structure, Assay 98%, mp78 - 81°C, Solubility: Methanol (Slightly), Water (Slightly, Sonicated), Storage: -20°C, D-(+)-Raffinose is a trisaccharide built from 1 mol each of D-galactose, D-glucose, and D-fructose which are obtained from it by acid hydrolysis. Invertase splits it into melibiose and saccharose. D-(+)-Raffinose occurs in Australian manna; in cottonseed meal. [1]. Suami, et al.: Carbohydr. Res., 26, 234 (1973), [2]. Rathbone, E.B., et al.: Dev. Food Carbohydr., 2, 145 (1980),
-Raffinose Pentahydrate.png)
Eriocitrin, (S)-3′,4′,5,7-Tetrahydroxyflavanone-7-[6-O-(α-L-rhamnopyranosyl)-β-D-glucopyranoside], Eriodictioside, Eriodictyol 7-O-rutinoside, C27H32O15 CAS NO.13463-28-0 MW596.53, form Solid, color Dark Beige to Very Dark Beige, NMR Conforms to Structure, MS Conforms to Structure, HPLC Assay 98.86% (220 nm), Solubility: DMSO (Slightly), Methanol (Slightly), Storage: -20°C, Eriocitrin is one of the main flavonoids in citrus limon byproduct dried powder (CBP) that exhibits antioxidant activity. [1]. Gargouri, B., et al.: Eur. Food Res. Technol., (2017)

(3R,4S,5R,6R)-6-(acetoxymethyl)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triyl triacetate, C30H35ClO11 CAS NO.461432-28-0 MW607.05, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 95.20%, Storage: Storage temp. 2-8°C
-6-(acetoxymethyl)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triyl triacetate.png)
(3R,4S,5R,6R)-6-(acetoxymethyl)-2-methoxy-2-(4-methyl-3-((5-phenylthiophen-2-yl)methyl)phenyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate, C33H36O10S MW624.70, Assay (NMR) 95%, Storage: Store at room temperature
-6-(acetoxymethyl)-2-methoxy-2-(4-methyl-3-((5-phenylthiophen-2-yl)methyl)phenyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate.png)
Fmoc-Thr[GalNAc(Ac)3-α-D]-OH, Fmoc-Thr(Ac₃AcNH-α-Gal)-OH, C33H38N2O13 CAS NO.116783-35-8 MW670.66, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp178-183°C, Storage: -80°C, 2 years; -20°C, 1 year; polypeptide, sealed storage, away from moisture
3-α-D]-OH.png)
α-D-Cellobiose octaacetate, alpha-D-Cellobiose octaacetate, C28H38O19 CAS NO.5346-90-7 MW678.59, Assay 95%, mp225-232 °C, bp683.055 °C at 760 mmHg, Storage: 2-8°C,

(1S,2S)-1-((2R,3R,4S,6S)-3-acetamido-4-acetoxy-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-2-yl)propane-1,2,3-triyl triacetate, C30H34BrClN2O14 CAS NO.153248-53-4 MW761.95, Assay 98%, Storage: 2-8°C
-1-((2R,3R,4S,6S)-3-acetamido-4-acetoxy-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-2-yl)propane-1,2,3-triyl triacetate.png)
Angoroside C, C36H48O19 CAS NO.115909-22-3 MW784.75, UNSPSC Code:41116107 Assay ≥95.0% (HPLC), Storage: storage temp. 2-8°C

MAC glucuronide linker-1, C42H47N3O17S CAS NO.2222981-71-5 MW897.90, Assay (NMR) 98%, Storage: 2-8°C, stored under nitrogen

gamma-Cyclodextrin C48H80O40 CAS NO.17465-86-0 MW1297.12, Beilstein:78740, EC Number:241-482-4, UNSPSC Code:12352005, form Solid, color White to Off-White, NMR Conforms to Structure, Elemental Analysis Conforms %C: 40.45, %H: 6.57, HPLC Assay 100.00% (ELSD), MS Conforms to Structure, Specific Rotation +152.1° (c = 0.5, Water), Solubility:DMSO (Slightly), Methanol(Slightly), Water (Slightly), Storage: 20°C, γ-Cyclodextrin is used as nanomolecular encapsulation for drug delivery and pharmaceuticals. Used in the synthesis of unilamellar vesicles. [1]. Ikeda, A. et al.: Chem. Comm., 50, 1288 (2014); Gudmundsdottir, B. et al.: J. Ocular Pharm. Ther., 30, 35 (2014);

Alpha-Cyclodextrin Hydrate, α-Cyclodextrin hydrate (1:x), C36H60O30.xH2O CAS NO.51211-51-9, Storage: Store at room temperature, form Solid, color White to off-white, Assay: ≥98.0%, mp278 °C, α-Cyclodextrin hydrate is used in the food industry. The structure of cyclodextrins allows it to form inclusion complexes with variety of molecules. It leads to enzymatic-like specificity in the reaction.
5-Deoxy-D-ribose, 5-deoxy-beta-D-ribofuranose, C5H10O4 CAS NO.13039-75-3 MW134.13, form Thick Oil, color Yellow to Dark Orange , bp294.5±19.0 °C at 760 mmHg, Solubility: Methanol (Sparingly), Water (Sparingly), Storage: +4°C, 5-Deoxy-D-ribose is a structural unit used in the synthesis of anticancer compounds. Used in the process for preparing Capecitabine and b-trialkyl carbonate nucleosides. [1]. Shimma, N., et al.: Bioorg. Med. Chem., 8, 1697 (2000),
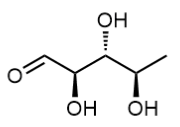
2-Deoxy-L-ribose, C5H10O4 CAS NO.18546-37-7 MW134.13, form Solid, color Light Yellow to Pale Beige, Assay (NMR) 98%, mp69-72 °C, bp167.23 °C at 760 mmHg, Solubility: Methanol (Slightly), Water (Slightly), Storage: 2-8°C, 2-Deoxy-L-ribose is an isomer of 2-Deoxy-D-ribose (D252000) which induces apoptosis by inhibiting the synthesis and increasing the efflux of glutathione. [1]. Aqeel Ahmed, et al. Colchicine glycorandomization influences cytotoxicity and mechanism of action. J Am Chem Soc. 2006 Nov 8;128(44):14224-5. [2]. Masao Shiozaki, et al. Synthesis and biological activity of hydroxylated analogues of KRN7000 (α-galactosylceramide). Carbohydr Res. 2013 Apr 5:370:46-66. [3]. Pauline Peltier-Pain, et al. Warfarin glycosylation invokes a switch from anticoagulant to anticancer activity. ChemMedChem. 2011 Aug 1;6(8):1347-50. [4]. Shin-ichi Akiyama, et al. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004 Nov;95(11):851-7. [5]. Yuichi Nakajima, et al. Inhibition of metastasis of tumor cells overexpressing thymidine phosphorylase by 2-deoxy-L-ribose. Cancer Res. 2004 Mar 1;64(5):1794-801. [6]. Fico, A., et al.: Free Radical Biology & Medicine, 45, 211 (2008)
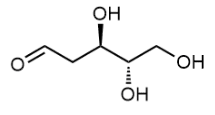
L-Arabinitol C5H12O5 CAS NO.7643-75-6 MW152.15, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0% , mp101-104 °C, bp194.6 °C at 760 mmHg, Solubility: DMSO (Slightly), Methanol (Slightly, Heated, Sonicated), Water (Slightly), Storage: Storage temp. 2-8°C, L-Arabitol is a rare sugar alcohol that is being investigated as a food additive that can reduce fat deposition in the intestines. L-Arabitol is used as an inducer for the expression of xylanase in Trichoderma reesei. L-Arabitol is used to identify, distinguish, and characterize L-arabitol dehydrogenase. L-Arabinitol is the stereoisomer of D-Arabinitol, both used as diagnostic tools for the detection of fungal infections in neutropenic patients. [1]. Shetty HU, et al. Cerebrospinal fluid and plasma distribution of myo-inositol and other polyols in Alzheimer disease. Clin Chem. 1996 Feb;42(2):298-302. [2]. Anna Walter, et al. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol Aging. 2004 Nov-Dec;25(10):1299-303. [3]. H U Shetty, et al. Cerebrospinal fluid and plasma distribution of myo-inositol and other polyols in Alzheimer disease. Clin Chem. 1996 Feb;42(2):298-302. [4]. Jian-ping Jia, et al. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer's disease and vascular dementia. Chin Med J (Engl). 2004 Aug;117(8):1161-4. [5]. J-M Serot, et al. Homocysteine and methylmalonic acid concentrations in cerebrospinal fluid: relation with age and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005 Nov;76(11):1585-7. [6]. Salonen, J.H., et al.: Eur. J. Clin. Microbiol. Infect. Dis., 20, 179 (2001); Sigmundsdottir, G. et al.: J. Clin. Microbiol., 38, 3039 (2000);

(3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyldihydrofuran-2(3H)-one, D-erythro-Pentonic acid, 2-deoxy-2-fluoro-2-methyl-, gamma-lactone, C6H9FO4 CAS NO.879551-04-9 MW164.13, form Solid, color Off-white to light brown, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.34%, OR(C=0.91g/100ml ACN): 149.7°, mp142-143 °C, bp352.7±42.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyldihydrofuran-2(3H)-one.png)
Methyl α-D-arabinofuranoside, Methyl Alpha-D-Arabinofuranoside, C6H12O5 CAS NO.56607-40-0 MW164.16, Assay (NMR) 98%, mp65-67 °C, bp348.7±42.0 °C at 760 mmHg

1-Amino-1-deoxy-β-D-galactose, β-D-Galactosylamine, C6H13NO5 CAS NO.74867-91-7 MW179.17, Assay (NMR) 98%, mp128-133 °C, [1]. Bo-Yu Li, et al. InCl(3)-catalyzed asymmetric aza-Friedel-Crafts reaction of indoles with imines generated from O-pivaloylated beta-D-galactosylamine. Carbohydr Res. 2010 Aug 16;345(12):1708-12. [2]. C F Mazitsos, et al. Galactosyl-biomimetic dye-ligands for the purification of Dactylium dendroides galactose oxidase. J Chromatogr A. 2002 Apr 19;954(1-2):137-50. [3]. N Monji, et al. Steric hindrance enzyme immunoassay (SHEIA); a novel method in enzyme immunoassay. Res Commun Chem Pathol Pharmacol. 1979 Oct;26(1):187-96.

Glucosamine, D-Glucosamine, Chitosamine, C6H13NO5 CAS NO.3416-24-8 MW179.17, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp88°C, Storage: Storage temp. 2-8°C
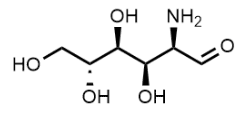
D-Glucamine, 1-Amino-1-deoxy-D-glucitol, C6H15NO5 CAS NO.488-43-7 MW181.19, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Purity (GC): 95.69%, Optical Rotation[a]: -7.9201°(C=1.0003g/100ml H2O), mp126-128°C, bp530.7°C at 760 mmHg, Solubility: Water (Slightly), Storage: Storage temp. 2-8°C
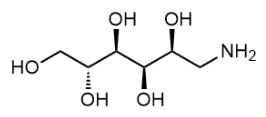
Dulcite, C6H14O6 CAS NO.608-66-2 MW182.17, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp188-189°C, bp275-280°C, Solubility: Methanol (Slightly), Water (Sparingly, Heated, Sonicated), Storage: Storage temp. 2-8°C, Dulcitol has antitumor activity. Dulcitol is the reduction product of Galactose. An increase in the level of Dulcitol is often a result of metabolism defect caused by a defect in galactose-1-phosphate uridylyltransferase (an autosomal recessive disorder). Dulcitol buildup can also lead to cataractogenesis. [1]. Alloussi S, et al. Effect of 5% galactose diet on galactose and dulcitol in plasma and lens of male and female pigs. Ann Nutr Metab. 1989;33(6):323-9. [2]. Alexandre F Somera, et al. Leaf-cutter ant fungus gardens are biphasic mixed microbial bioreactors that convert plant biomass to polyols with biotechnological applications. Appl Environ Microbiol. 2015 Jul;81(13):4525-35. [3]. Anna Walter, et al. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol Aging. 2004 Nov-Dec;25(10):1299-303. [4]. H U Shetty, et al. Cerebrospinal fluid and plasma distribution of myo-inositol and other polyols in Alzheimer disease. Clin Chem. 1996 Feb;42(2):298-302. [5]. K J B?r, et al. Pentosidine and N(epsilon)-(carboxymethyl)-lysine in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2003 Mar-Apr;24(2):333-8. [6]. Ning, C. et al.: Pediat. Res., 48, 211 (2000); [7]. Cheng, H. et al.: Exp. Eye Res., 51, 345 (1990); [8]. Wells, W.W. et al.: Galactosemia, 227 (1969);

(3aR,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-ol,2-O,3-O-Isopropylidene-D-ribo-pentofuranose, C8H14O5 CAS NO.4099-88-1 MW190.19, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (GC): 98.46%, bp337.4±42.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-ol.png)
Methyl α-D-mannopyranoside, α-Methyl-D-mannoside, C7H14O6 CAS NO.617-04-9 MW194.18, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.04%, Residue on Ignition: 0.24%, Optical Rotation: 74.6°(C=0.01g/ml H2O), mp193-196 °C, bp250.62 °C at 760 mmHg, Solubility: Water (Slightly), Storage: Storage temp. 2-8°C, Methyl α-D-mannopyranoside is a competitive inhibitor of E. coli binding mannose. It is also used as pharmaceutical raw materials healthcare products and intermediates. It is also used as animal drugs. Methyl a-D-Mannopyranoside is used in preparation of Glucose-responsive insulin conjugates. [1]. Maretti E, et, al. Surface engineering of Solid Lipid Nanoparticle assemblies by methyl α-d-mannopyranoside for the active targeting to macrophages in anti-tuberculosis inhalation therapy. Int J Pharm. 2017 Aug 7; 528(1-2):440-451. [2]. Julia Deschamp, et al. Towards a stable noeuromycin analog with a D-manno configuration: synthesis and glycosidase inhibition of D-manno-like tri- and tetrahydroxylated azepanes. Bioorg Med Chem. 2012 Jan 15;20(2):641-9. [3]. Karen T Welch, et al. Rational design of novel glycomimetics: inhibitors of concanavalin A. Bioorg Med Chem Lett. 2008 Dec 15;18(24):6573-5. [4]. M Aronson, et al. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329-32. [5]. Oliver Schwardt, et al. Design, synthesis and biological evaluation of mannosyl triazoles as FimH antagonists. Bioorg Med Chem. 2011 Nov 1;19(21):6454-73. [6]. Zhu, Y., et al.; PCT Int. Appl., (2020)

2-Azido-2-deoxy-D-galactose, C6H11N3O5 CAS NO.68733-26-6 MW205.17, Assay (NMR) 98%

Allyl α-D-glucopyranoside, C9H16O6 CAS NO.7464-56-4 MW220.22, Assay (NMR) 98%, mp138-141 °C, bp415.0±45.0 °C at 760 mmHg

Ethyl 1-thio-β-D-glucopyranoside, Ethyl β-Thioglucopyranoside, C8H16O5S CAS NO.7473-36-1 MW224.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp87-89 °C, bp440.8±45.0 °C at 760 mmHg, Solubility: Dichloromethane, Methanol, Storage: Storage temp. 2-8°C, [1]. Komba, S., et al.: Bioorg. Med. Chem., 4, 1833 (1996), [2]. Bertozzi, C., et al.: Science, 291, 2357 (2001), [3]. Davis, B., et al.: Chem. Rev., 102, 579 (2002),

Ethyl-α-D-thioglucopyranoside, Ethyl α-thioglucopyranoside, C8H16O5S CAS NO.13533-58-9 MW224.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp156 °C, bp440.8±45.0 °C at 760 mmHg, Solubility: DMSO (Slightly), Methanol (Slightly), Water (Slightly)Storage: Storage temp. 2-8°C, [1]. Komba, S., et al.: Bioorg. Med. Chem., 4, 1833 (1996), Bertozzi, C., et al.: Science, 291, 2357 (2001), Davis, B., et al.: Chem. Rev., 102, 579 (2002),

Phenyl β-D-glucopyranoside, (2R,3S,4S,5R,6S)-2-(Hydroxymethyl)-6-phenoxy-tetrahydro-2H-pyran-3,4,5-triol, C12H16O6 CAS NO.1464-44-4 MW256.25, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 99.58%, mp176-178°C, bp482.1 °C at 760 mmHg, Solubility: Methanol (Slightly), Water (Slightly, Sonicated), Storage: 2-8°C, sealed storage, away from moisture, [1]. Hwang SJ, et al. Phenyl-β-D-Glucopyranoside Exhibits Anti-inflammatory Activity in Lipopolysaccharide-Activated RAW 264.7 Cells. Inflammation. 2015;38(3):1071-9.
1,2:5,6-Di-O-isopropylidene-α-D-gulofuranose, C12H20O6 CAS NO.14686-89-6 MW260.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.09%, mp110-111 °C, bp362.8±42.0 °C at 760 mmHg, Solubility: Chloroform (Slightly), Methanol (Slightly), Storage: Storage temp. 2-8°C, 1,2:5,6-Di-O-isopropylidene-a-D-gulofuranose was a useful reagent in the preparation of fluorinated UDP-galactofuranose probes. [1]. Zhang, Q., et al.: J. Am. Chem. Soc., 123, 6756 (2001);
2-[(Azidoacetyl)amino]-2-deoxy-D-glucose, C8H14N4O6 CAS NO.92659-90-0 MW262.22, Assay (NMR) 98%, 2-[(Azidoacetyl)amino]-2-deoxy-D-glucose is a azide-labeled sugar that is widely used for studying glycoproteins through in vivo metabolic labeling and chemostelective ligation. [1]. Li, Y.H., et al.: Molecules., 16, 6396 (2011); [2]. Gurcel, C., et al.: Anal. Bioanal. 390, 2089 (2008); [3]. Zou, W., et al.: Bioorg. Med. Chem. Lett., 22, 3540 (2012);
amino]-2-deoxy-D-glucose.png)
4-Nitrophenyl β-D-xylopyranoside, C11H13NO7 CAS NO.2001-96-9 MW271.22, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.60%, mp160 °C, bp523.2±50.0 °C at 760 mmHg, Solubility: Chloroform, Methanol (Sparingly), Water (Slightly, Heated), Storage: -20°C, protect from light, stored under nitrogen, Substrate for the β-D-xylosidase colorimetric assay. Also shown to degrade protein polysaccharides in vivo. [1]. Herrmann MC, et, al. The beta-D-xylosidase of Trichoderma reesei is a multifunctional beta-D-xylan xylohydrolase. Biochem J. 1997 Jan 15;321 ( Pt 2)(Pt 2):375-81. [2]. Bruna Silveira Lamanes dos Santos, et al. Thermotolerant and mesophylic fungi from sugarcane bagasse and their prospection for biomass-degrading enzyme production. Braz J Microbiol. 2015 Jul 1;46(3):903-10. [3]. Douglas B Jordan, et al. Aminoalcohols as probes of the two-subsite active site of beta-D-xylosidase from Selenomonas ruminantium. Biochim Biophys Acta. 2009 Jan;1794(1):144-58. [4]. G Fagnen, et al. Activation of protein kinase C increases proteoglycan synthesis in immature rat Sertoli cells. Biochim Biophys Acta. 1999 Oct 18;1472(1-2):250-61. [5]. J Zhou, et al. Beta-xylosidase activity of a GH3 glucosidase/xylosidase from yak rumen metagenome promotes the enzymatic degradation of hemicellulosic xylans. Lett Appl Microbiol. 2012 Feb;54(2):79-87. [6]. Jordan, D., et al.: App. Biochem. Biotechnol., 146, 137 (2008), Li, X., et al.: App. Env. Microbiol., 74, 7482 (2008),

4-Aminophenyl-β-D-galactopyranoside, 4-Aminophenyl-beta-D-galactopyranoside, C12H17NO6 CAS NO.5094-33-7 MW271.27, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp163 °C, bp555.9 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Ardhendu K Mandal, et al. Sugar coated liposomal flavonoid: a unique formulation in combating carbontetrachloride induced hepatic oxidative damage. J Drug Target. 2005 Jun;13(5):305-15. [2]. D M Belen'ki?, et al. [Chemical attachment of p-aminophenylgalactoside to acid alpha-glucosidase from human liver]. Biokhimiia. 1982 Jul;47(7):1141-6. [3]. D P Sarkar, et al. Effect of membrane composition on the immune reactivity of galactosylated liposomes. Indian J Biochem Biophys. 1984 Jun;21(3):155-7. [4]. D P Sarkar, et al. Immunogenicity of galactosylated liposomes. Immunol Commun. 1984;13(1):5-13. [5]. Daniel Bello-Gil, et al. Specific and reversible immobilization of proteins tagged to the affinity polypeptide C-LytA on functionalized graphite electrodes. PLoS One. 2014 Jan 31;9(1):e87995.

4-Aminophenyl-α-D-mannopyranoside, 4-Aminophenylmannosid, C12H17NO6 CAS NO.34213-86-0 MW271.27, form Solid, color Off-White to Beige, Assay (NMR) 98%, mp165-166 °C, bp555.9±50.0 °C at 760 mmHg, Solubility: DMSO (Slightly), Methanol (Slightly), Storage: -20°C, 4-Aminophenyl-α-D-pyranosyl mannose is a substrate of pyranosyl mannose, as a rapid molecular diagnostic method used to detect the absorbance of p-nitrophenol generated by the enzyme hydrolyzing 4-aminophenyl-α-Dpyranosyl mannose by spectrophotometry, to confirm its enzymatic activity, and to judge whether there is an increase or decrease in the activity of pyosyl mannose, and whether the enzyme is absent or not. Increases the uptake rate of liposomes. [1]. Haisheng Peng, et al. Liposomes modified with p-aminophenyl-α-D-mannopyranoside: a promising delivery system in targeting the brain. Ther Deliv. 2013 Dec;4(12):1475-7. [2]. K Shimura, et al. Affinophoresis of pea lectin and fava bean lectin with an anionic affinophore, bearing rho-aminophenyl-alpha-D-mannoside as an affinity ligand. J Chromatogr. 1987 Jul 29:400:353-9. [3]. K Shimura, et al. Capillary affinophoresis of pea lectin with polyliganded affinophores: a model study of divalent-polyvalent interactions. Electrophoresis. 1998 Mar;19(3):397-402. [4]. K Shimura, et al. Determination of the affinity constants of pea lectin for neutral sugars by capillary affinophoresis with a monoligand affinophore. J Biochem. 1996 Dec;120(6):1146-52. [5]. M Triggiani, et al. Secretory phospholipases A2 induce beta-glucuronidase release and IL-6 production from human lung macrophages. J Immunol. 2000 May 1;164(9):4908-15. [6]. Chono, S., et al.: J. Pharm., Pharmacol., 59, 75 (2007),

D-Ribose 5-phosphate disodium salt hydrate, C5H9Na2O8P·xH2O CAS NO.18265-46-8 MW274.07 (anhydrous basis), form Powder, color white, Assay ≥98% (TLC), impurities 6.0-15.0% water (Karl Fischer), solubility water: 50 mg/mL, clear, colorless, Storage: storage temp. −20°C, Ribose-5-phosphate is an intermediate of the oxidative branch of the Pentose Phosphate Pathway(PPP) and an end product of the nonoxidative branch of the PPP. Ribose-5-phosphate is used in the synthesis of nucleotides and nucleic acids.
p-Methoxyphenyl b-D-galactoside, 4-Methoxyphenyl beta-D-Galactopyranoside, C13H18O7 CAS NO.3150-20-7 MW286.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥95.0%, mp161°C, bp519°C at 760 mmHg, Storage: Storage temp. -20°C
Indoxyl β-D-galactopyranoside, C14H17NO6 CAS NO.126787-65-3 MW295.29, form Powder, Assay (NMR) 99.5%, impurities ≤1 mol/mol acetone, application(s) diagnostic assay manufacturing, hematology, histology, bp606.7 °C at 760 mmHg, Storage: 2-8°C
4-Nitrophenyl β-D-mannopyranoside, p-Nitrophenyl β-D-Mannopyranoside, C12H15NO8 CAS NO.35599-02-1 MW301.25, form Solid, color White to Off-White, Assay (NMR) 98%, mp207-209 °C, bp582.2±50.0 °C at 760 mmHg, Solubility: DMSO, Methanol, Water, [1]. Bjoern Chapuy, et al. ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug. Haematologica. 2009 Nov;94(11):1528-36. [2]. G W Forsyth, et al. Variable expression of leukocyte cytosolic broad-specificity beta-glucosidase activity. Clin Chim Acta. 1993 Jul 16;216(1-2):11-21. [3]. Giuseppina Andreotti, et al. Purification and characterization of a beta-D-mannosidase from the marine anaspidean Aplysia fasciata. J Biotechnol. 2005 Sep 22;119(1):26-35. [4]. P J Healy, et al. Alternative substrates for use in the detection of goats heterozygous for beta-mannosidosis. Res Vet Sci. 1982 Jul;33(1):73-5. [5]. S H Khan, et al. Synthesis of some D-mannosyl-oligosaccharides containing the methyl and 4-nitrophenyl beta-D-mannopyranoside units. Carbohydr Res. 1990 Oct 15;207(1):57-69. [6]. Yang, L., et al.: J. Agric. Food Chem., 52, 1940 (2004),

Nonyl β-D-glucopyranoside, C15H30O6 CAS NO.69984-73-2 MW306.40, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Optical Rotation: Consistent with structure, Assay (NMR): ≥98.0%, OR(C=0.68700g/100ml DCM): -34.3°, mp70 °C, bp465.3 °C at 760 mmHg, Storage: Storage temp. 2-8°C, n-nonyl-β-D-glucoside is a nonionic (3) surfactant. Nonyl glucoside a dialyzable nonionic detergent used for functional solubilization and purification of membrane proteins. [1]. Annette Meister, et al. The interaction of n-nonyl-β-d-glucopyranoside and sodium dodecyl sulfate with DMPC and DMPG monolayers studied by infrared reflection absorption spectroscopy. Phys. Chem. Chem. Phys., 2004,6, 5543-5550 [2]. Parmjeet Randhawa, et al. BK Virus replication in vitro: limited effect of drugs interfering with viral uptake and intracellular transport. Antimicrob Agents Chemother. 2007 Dec;51(12):4492-4.

8-Quinolinyl β-D-galactopyranoside, C15H17NO6 CAS NO.113079-84-8 MW307.30, Assay (NMR) 95%, bp582.5±50.0 °C at 760 mmHg, Storage: 2-8°C,

Indoxyl β-D-glucuronide cyclohexylammonium salt, C14H15NO7·C6H13N CAS NO.35804-66-1 MW408.45, form Powder, color Colorless or White, Assay ≥98%(HPLC), optical activity [α]20/D -80.0±5.0, Solubility (Color) Colorless to Very Faint Yellow and Colorless to Very Faint Green-Yellow, Solubility (Turbidity) Clear, [1]. [Evaluation of a new disk containing indoxyl-beta-D-glucuronide for rapid identification of Escherichia coli]. S Fujita et al. Rinsho byori. The Japanese journal of clinical pathology, 44(9), 895-898 (1996-09-01) To establish a simple identification procedure for Escherichia coli, we developed a disk containing indoxyl-beta-D-glucuronide, the chromogenic substrate of beta-D-glucuronidase. Of 188 isolates of Enterobacteriaceae, 101 (97%) of 104 strains of E. coli and two (67%) of three strains of [2]. Indoxyl-beta-D-glucuronide and 3-indoxyl sulfate in plasma of hemodialysis patients. S Agatsuma et al. Clinical nephrology, 45(4), 250-256 (1996-04-01) The content of indoxyl-beta-D-glucuronide, which has been found in patients' plasma as a new indicator of renal failure, logarithmically correlated with that of 3-indoxyl sulfate (indican) in the plasma of hemodialysis patients, showing another weak correlation with beta(2)-microglobulin content. The [3]. Rapid and specific detection of hydroxyl radical using an ultraweak chemiluminescence analyzer and a low-level chemiluminescence emitter: application to hydroxyl radical-scavenging ability of aqueous extracts of Food constituents. C H Tsai et al. Journal of agricultural and food chemistry, 49(5), 2137-2141 (2001-05-23) With the availability of an ultraweak chemiluminescence analyzer, it is possible to monitor the production of a specific oxygen-derived reactive species, such as hydroxyl radical ((*)OH), whenever a suitable chemiluminescent probe is obtainable. Reported herein is the development of a [4]. Accumulation of indoxyl-beta-D-glucuronide in uremic serum: suppression of its production by oral sorbent and efficient removal by hemodialysis. T Niwa et al. Nephron, 74(1), 72-78 (1996-01-01) We identified and quantified indoxyl-beta-D-glucuronide in uremic serum and urine to determine the metabolism of indoles including indoxyl sulfate in uremic patients. Serum levels of indoxyl-beta-D-glucuronide were markedly increased in undialyzed uremic patients, in patients on hemodialysis, and in patients [5]. Evaluation of indoxyl-beta-D-glucuronide as a chromogen in media specific for Escherichia coli. J R Haines et al. Applied and environmental microbiology, 59(8), 2758-2759 (1993-08-01) Indoxyl-beta-D-glucuronide (indoxyl) was evaluated as a specific chromogen for detection of Escherichia coli by the membrane filter method. In all, 413 colonies were tested from the indoxyl-supplemented media, yielding 93.3% confirmation, as E. coli. Compared with the indoxyl medium, other [6]. Rapid detection of Escherichia coli in urine samples by a new chromogenic beta-glucuronidase assay. G J Delisle et al. Journal of clinical microbiology, 27(4), 778-779 (1989-04-01) A new compound, indoxyl-beta-D-glucuronide, was assessed as a substrate for the rapid detection of Escherichia coli in urine. Incorporation of this compound into MacConkey agar allowed the direct differentiation of E. coli as deep blue colonies distinct from lactose and [7]. Comparison of the recoveries of Escherichia coli and total coliforms from drinking water by the MI agar method and the U.S. Environmental Protection Agency-approved membrane filter method. K P Brenner et al. Applied and environmental microbiology, 62(1), 203-208 (1996-01-01) Drinking water regulations under the Final Coliform Rule require that total coliform-positive drinking water samples be examined for the presence of Escherichia coli or fecal coliforms. The current U.S. Environmental Protection Agency-approved membrane filter (MF) method for E. coli requires [8]. Indoxyl-beta-D-glucuronide, the primary emitter of low-level chemiluminescence in plasma of hemodialysis patients. S Agatsuma et al. Clinical chemistry, 40(8), 1580-1586 (1994-08-01) Characteristic light emission induced by the oxidation of hydroxyl radicals has been found in plasma of hemodialysis patients (Agatsuma et al., Clin Chem 1992;38:48-55). We purified a primary emitter, a chemiluminescent component peaking at 430 nm, by anion-exchange chromatography and
Glucovanillin, Vanillin 4-O-beta-D-Glucoside, Avenein, Vanillin glucoside, Vanilloside, C14H18O8 CAS NO.494-08-6 MW314.29, form Powder, color White, NMR Conforms to Structure, MS Conforms to Structure, Assay ≥99.800% (HPLC 230nm), Solubility:DMSO (Slightly), Water(Slightly), application(s) food and beverages pharmaceutical, Storage: storage temp. 2-8°C, Vanillin 4-O-b-D-Glucoside is the glucosylated precursor of Vanillin found in the seed pods of Vanilla planifola. During the curing process Vanillin 4-O-b-D-Glucoside is transformed into the aromatic vanillin. [1]. Odoux, E. et al.: Fruits, 61, 171 (2006); [2]. Palama, T.L. et al.: Environ. Exp. Bot., 72, 258 (2011); [3]. Dunphy, P. et al.: Perf. Flav., 34, 34 (2009);
1,2,3,4-Tetra-O-acetyl-β-D-xylopyranose, 1,2,3,4-Tetra-O-acetyl-beta-D-xylopyranose, C13H18O9 CAS NO.4049-33-6 MW318.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Optical Rotation[α]: -24.534°(C=0.01g/ml CHCL3), mp123-125 °C, bp362.9±42.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C

beta-D-Ribofuranose 1,2,3,5-tetraacetate,C13H18O9 CAS NO.13035-61-5 MW318.28,form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp81-83°C, bp385.6°C at 760 mmHg, Solubility:Chloroform (Slightly),Methanol (Slightly), Storage: Storage temp. 2-8°C, Used in the synthesis of 3-(b-D-ribofuranosyl)-2,3-dihydro-6H-1,3-oxazine-2,6-dione, a new pyrimidine nucleoside analog related to uridine. [1]. Furukawa, Y., and Honjo, MA novel method for the synthesis of purine nucleotides using Friedel-Crafts catalysts Chem. Pharm. Bull. (Tokyo) 16(6) 1076-1080(1968). [2]. Wicke, L., Engels, JW, Gambari, R., et al. Synthesis and antiproliferative activity of quinolone nucleotides against the human myelogenous leukemia k-562 cell line Arch. Pharm. (Weinheim) 346(10)757-765(2013). [3]. Chwang, T.-L., et al.: J. Med. Chem., 19, 643 (1976),

(3R,4R,5R)-5-(Acetoxymethyl)tetrahydrofuran-2,3,4-triyl triacetate, C13H18O9 CAS NO.28708-32-9 MW318.28, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, Water(KF): 0.23%, OR(C=0.50g/100ml MEOH): -17.0°, mp82 °C, bp385.6°C at 760 mmHg, Storage: Storage temp. 2-8°C
-5-(Acetoxymethyl)tetrahydrofuran-2,3,4-triyl triacetate.png)
N-Acetyl-β-neuraminic acid methyl ester, N-Acetyl-Neuraminic acid (Sialic acid), C12H21NO9 CAS NO.22900-11-4 MW323.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=0.65245 MEOH): -23.7°, mp193.5-194.5 °C, bp693.8±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Organic and Biomolecular Chemistry, vol 10, #5 p. 931-934

Undecyl β-D-glucopyranoside, C17H34O6 CAS NO.70005-86-6 MW334.45, Assay (NMR) 98%, bp487.8±45.0 °C at 760 mmHg, [1]. Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, vol. 40, # 9 p. 796-801,
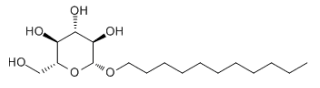
Dipotassium glucose-6-phosphate, C6H11K2O9P CAS NO.5996-17-8 MW336.32, Assay (NMR) 98%

4-Nitrophenyl β-D-glucuronide (sodium), p-Nitrophenyl β-Glucuronic Acid Sodium Salt, 4-Nitrophenyl β-D-Glucopyranosiduronic AcidSodium Salt, C12H12NNaO9 CAS NO.89772-41-8 MW337.21, form Solid, color Pale Yellow to Yellow, NMR Conforms to Structure, MS Conforms to Structure, Assay (NMR) 98%, mp150-153 °C, Solubility:DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated), Water (Slightly), Storage: -20°C, [1]. Muraoka, M., et al.: J. Biol. Chem., 282, 24615 (2007); [2]. De Angelis, M., et al.: Vet. Microbiol., 123, 133 (2007); [3]. Geddie, M., et al.: J. Mol. Biol., 369, 1052 (2007); [4]. Ball, A., et al.: J. Enz. Inhib. Med. Chem., 23, 131 (2008);
.png)
4-Nitrophenyl-N-acetyl-α-D-galactosaminide, pNP-GalNAc, C14H18N2O8 CAS NO.23646-68-6 MW342.30, form Solid, color White to Light Yellow, Assay (NMR) 97%, mp229-231 °C, bp694.7±55.0 °C at 760 mmHg, Solubility: DMSO (Slightly), Methanol (Slightly, Sonicated), Storage: -20°C, 4-Nitrophenyl-N-acetyl-α-D-galactosamine can be used as a chromogenic synthetic substrate for theymatic determination of lysosomal enzyme α-N-acetylgalactosaminidase. After cleavage, the substrate forms a yellow solution of p-nitrophenol, which can be measured at 400nm, pH 10.6. [1]. E Kamst, et al. Chitin oligosaccharide synthesis by rhizobia and zebrafish embryos starts by glycosyl transfer to O4 of the reducing-terminal residue. Biochemistry. 1999 Mar 30;38(13):4045-52. [2]. H De Boeck, et al. Binding of simple carbohydrates and some of their chromophoric derivatives to soybean agglutinin as followed by titrimetric procedures and stopped flow kinetics. J Biol Chem. 1984 Jun 10;259(11):7067-74. [3]. Hong-Ming Chen, et al. Syntheses of the 3- and 4-thio analogues of 4-nitrophenyl 2-acetamido-2-deoxy-beta-D-gluco- and galactopyranoside. Carbohydr Res. 2007 Nov 5;342(15):2212-22. [4]. I Pócsi, et al. Studies on the N-acetyl-beta-D-hexosaminidase B from germinating Lupinus luteus L. seeds. I. Purification and characterization. Biochim Biophys Acta. 1990 May 31;1039(1):110-8. [5]. Jin-Jin Xie, et al. Inactivation kinetics of beta-N-acetyl-D-glucosaminidase from green crab (Scylla serrata) in dioxane solution. J Biomol Struct Dyn. 2009 Feb;26(4):509-15.
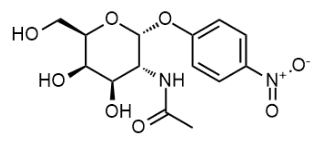
Sodium (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(quinolin-8-yloxy)tetrahydro-2H-pyran-2-carboxylate, 8-Hydroxyquinoline-β-D-glucuronide sodium salt, C15H14NNaO7 CAS NO.207728-71-0 MW343.27, Assay (NMR) 97%,
-3,4,5-trihydroxy-6-(quinolin-8-yloxy)tetrahydro-2H-pyran-2-carboxylate.png)
1,2,3,4-Tetra-O-acetyl-β-D-glucopyranose, 1,2,3,4-Tetra-O-acetyl-beta-D-glucopyranos, C14H20O10 CAS NO.13100-46-4 MW348.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=0.685g/100ml CHCL3 ): 8.7°, mp126-128 °C, bp418.4±45.0 °C at 760 mmHg, Solubility: Chloroform (Slightly), Water (Slightly), Storage: Storage temp. 2-8°C, It was confirmed that the phosphorylated derivative was valuable in the study of the substrate of myo-inositol synthesizing enzyme and in the preparation of an surfactants. [1]. Graham, B., et al.: Tetrahedron, 47, 3895 (1991); [2]. Alain, M., et al.: Carbohydrate Res., 229, 323 (1992)

1,3,4,6-Tetra-O-acetyl-β-D-mannopyranose, C14H20O10 CAS NO.18968-05-3 MW348.30, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR[α](C=0.65/100ml CHCL3): -21.8°, mp160-161 °C, bp425.5±45.0 °C at 760 mmHg, Storage: Store at room temperature, An intermediate in the preparation of precursors of 2-[18F]fluoro-2-deoxy-D-glucose for diagnostic use in positron emission tomography. [1]. Pozsgay Glaudemans Robbins Schneerson, Tetrahedron, 1992, vol. 48, # 47 p. 10249-10264, [2]. US2006/67888 A1, Page/Page column 11 sheet 7, [3]. Chemische Berichte, vol. 63, p. 386,390, [4]. Lichtenthaler, Frieder W. Jarglis, Pan Chemische Berichte, 1980, vol. 113, # 2 p. 489-510, [5]. Maschauer, S. et al.: J. Lab. Comp. Radiopharmac., 48, 701 (2005);

4-Acetamidophenyl Beta-D-Glucuronide Sodium Salt, Acetaminophen Glucuronide Sodium Salt, Paracetamol Glucuronide Sodium Salt, C14H16NNaO8 CAS NO.120595-80-4 MW349.27, form Solid, color White to Pale Red, NMR Conforms to Structure Conforms, MS Conforms to Structure, Assay (PHLC 245nm) 99.84%, Solubility: Methanol (Slightly), Water(Slightly), Storage: -20°C, 4-Acetamidophenyl b-D-Glucuronide is a major urinary metabolite of Acetaminophen (Paracetamol) in human (1). Acetaminophen is an analgesic and antipyretic agent and used as a pain reliever to treat headache, muscle aches, and arthritis (2,3). [1]. Johnston, D., et al.: J. Chem. Res., Synop., 12, 406 (1988), [2]. McGill, M. R. and Jaeschke, H. Pharm Res. 30, 2174 (2013), [3]. Fairbrother, J.E., et al.: Anal. Profiles Drug Subs., 3, 1 (1974)
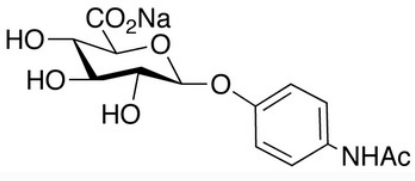
Ethyl 2-deoxy-2-phthalimido-β-D-thioglucopyranoside, C16H19NO6S CAS NO.130539-43-4 MW253.39, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR(C=0.71450 CHCL3 ): 8.2°, bp594.3±50.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C
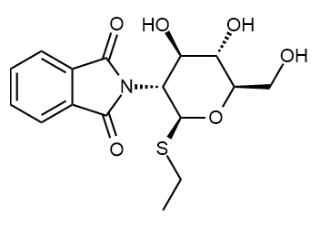
CAS NO.117405-51-3

4-S-β-D-Glucopyranosyl-4-thio-D-glucose, C12H22O10S CAS NO.80951-92-4 MW358.36, Assay (NMR) 97%, [1]. D Rho, et al. Induction of cellulose in Schizophyllum commune: thiocellobiose as a new inducer. J Bacteriol. 1982 Jan;149(1):47-53. [2]. E Montero, et al. NMR studies of the conformation of thiocellobiose bound to a beta-glucosidase from Streptomyces sp. FEBS Lett. 1998 Jan 16;421(3):243-8. [3]. G Henriksson, et al. Substrate specificity of cellobiose dehydrogenase from Phanerochaete chrysosporium. Biochim Biophys Acta. 1998 Mar 3;1383(1):48-54. [4]. Meera E Atreya, et al. Alleviating product inhibition in cellulase enzyme Cel7A. Biotechnol Bioeng. 2016 Feb;113(2):330-8. [5]. Pablo Isorna, et al. Crystal structures of Paenibacillus polymyxa beta-glucosidase B complexes reveal the molecular basis of substrate specificity and give new insights into the catalytic machinery of family I glycosidases. J Mol Biol. 2007 Aug 31;371(5):1204-18.

(4S,5R)-4-[(tert-Butyldimethylsilyl)oxy]-5-{[(tert-butyldimethylsilyl)oxy]methyl}oxolan-2-one, 3,5-Di-O-(tert-butyldimethylsilyl)-2-deoxy-D-ribonolactone, C17H36O4Si2 CAS NO.83159-91-5 MW360.64, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, OR[α](C=0.11 g/100ml CHCL3): 14.1°, Storage: Store at room temperature, keep dry and cool, [1] Kozikowski, Alan P. Ghosh, Arun K. Journal of Organic Chemistry, 1984, vol. 49, # 15 p. 2762-2772, [2]. Agricultural and Biological Chemistry, vol. 49, # 5 p. 1435-1440, [3]. Journal of Organic Chemistry, vol. 72, # 10 p. 3945-3948
-4-[(tert-Butyldimethylsilyl)oxy]-5-{[(tert-butyldimethylsilyl)oxy]methyl}oxolan-2-one.png)
Ethyl 2,3,4-tri-O-acetyl-β-D-glucuronide methyl ester, C15H22O10 CAS NO.77392-66-6 MW362.33, Assay (NMR) 98%, mp102-103 °C, Solubility: Chloroform, Methanol, Storage: +4°C, a compound useful in organic synthesis.
2,3,4,6-tetra-O-acetyl-β-D-chloroglucose, β-L-Glucopyranosyl chloride, 2,3,4,6-tetraacetate, C14H19ClO9 CAS NO.66966-08-3 MW366.75, Assay (NMR) 98%
2,3,4,6-Tetra-O-acetyl-β-D-galactopyranosyl azide, C14H19N3O9 CAS NO.13992-26-2 MW373.32, Assay (NMR) 98%, mp96-99 °C

2,3,4,6-Tetra-O-acetyl-α-D-mannopyranosyl azide, C14H19N3O9 CAS NO.53784-29-5 MW373.32, Assay (NMR) 98%, mp103-105 °C

(2R,3R,4S,5S,6S)-6-(Methoxycarbonyl)tetrahydro-2H-pyran-2,3,4,5-tetrayl tetraacetate, Methyl(1,2,3,4-tetra-O-acetyl-.alpha.-D-glucopyranosid)uronate, C15H20O11 CAS NO.5432-32-6 MW376.31, Assay (NMR) 95%, mp108-120 °C, bp412.3±45.0 °C at 760 mmHg
-6-(Acetoxymethyl)tetrahydro-2H-pyran-2,3,4,5-tetrayl tetraacetate.png)
Loganic Acid, C16H24O10 CAS NO.22255-40-9 MW376.36, form Solid, color Off-White, Assay ≥95% (LC/MS-ELSD), Solubility: Aqueous Base (Slightly), DMSO (Slightly), Methanol (Slightly), Storage: 2-8℃, Loganic Acid is found to be present in both the fermented fruits and brines. Exhibits antioxidant activity. Loganic acid is a naturally occurring iridoid monoterpeneglusoside. It is a key constituent of Swertia caroliniensis. Loganic acid is involved in the biosynthesis of indole alkaloids in Vinca rosea. [1]. Czyzowska, A., et al.: J. Food Sci. Technol., (2017); [2]. Kucharska, A. Z., et al.: Mol., 22, 405/1-405/20 (2017)

DEAE-Cellulose, Diethylaminoethyl cellulose, C6H15NO.xUnspecified CAS NO.9013-34-7, form Solid, color White to off-white, Assay: ≥97.0%, Storage: 2-8°C, protect from light, A cellulose Ion-exchange adsorbent., [1]. Alessandra Olianas, et al. Striped mullet (Mugil cephalus) hemoglobin system: multiplicity and functional properties. J Comp Physiol B. 2011 Feb;181(2):187-97. [2]. D Z Ma, et al. A peptide with potent antifungal and antiproliferative activities from Nepalese large red beans. Peptides. 2009 Dec;30(12):2089-94. [3]. Edita Mazoniene, et al. Interaction of cellulose-based cationic polyelectrolytes with mucin. Colloids Surf B Biointerfaces. 2011 Mar;83(1):160-4. [4]. Ines Maalej, et al. Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus: purification and characterization. Appl Biochem Biotechnol. 2009 Jul;158(1):200-12. [5]. Janez ??an?ar, et al. Ni speciation in tea infusions by monolithic chromatography--ICP-MS and Q-TOF-MS. Anal Bioanal Chem. 2013 Feb;405(6):2041-51.

1,3,4,6-Tetra-O-acetyl-2-amino-2-desoxy-β-D-glucopyranose (hydrochloride), C14H22ClNO9 CAS NO.10034-20-5 MW383.78, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, mp197-200°C, bp429.5ºC at 760 mmHg, Solubility: Methanol (Slightly), Water (Slightly), Storage: Storage temp. -20°C, A potential metabolic inhibitor of cellular-membrane glycoconjugates. [1]. Mark, B., et al.: J. Biol. Chem., 276, 10330
.png)
(2S,3S,4S,5R,6R)-6-(Acetoxymethyl)tetrahydro-2H-pyran-2,3,4,5-tetrayl tetraacetate, 1,2,3,4,6-Penta-O-acetyl-b-D-mannopyranose, C16H22O11 CAS NO.4026-35-1 MW390.34, form Solid, color White to Off-White, Assay (NMR) 98%, Solubility: Chloroform (Slightly), Methanol (Slightly), Storage: 2-8°C
-6-(Acetoxymethyl)tetrahydro-2H-pyran-2,3,4,5-tetrayl tetraacetate.png)
D-Glucose-6-phosphate (dipotassium hydrate), C6H17K2O12P CAS NO.207727-36-4 MW390.36, Assay (NMR) 98%
.png)
dimethyl 2-((4R,5S)-5-(2-(benzyloxy)acetyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-2-oxoethylphosphonate, C18H25O8P CAS NO.89291-74-7 MW400.36, Assay (NMR) 98%,
-5-(2-(benzyloxy)acetyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-2-oxoethylphosphonate.png)
Acetobromo-α-D-galactose, 2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide, C14H19BrO9 CAS NO.3068-32-4 MW411.20, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Water(KF): 0.19%, mp83-85 °C, bp412.0±45.0 °C at 760 mmHg, Storage: Storage temp. -20°C, 2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide is an intermediate in the synthesis of the antiviral flavonoid, nepetalactone A. [1]. Ahmed I Khodair, et al. A new approach for the N- and S-galactosylation of 5-arylidene-2-thioxo-4-thiazolidinones. Carbohydr Res. 2011 Dec 27;346(18):2831-7. [2]. Bart Ruttens, et al. Synthesis of spacer-equipped phosphorylated di-, tri- and tetrasaccharide fragments of the O-specific polysaccharide of Vibrio cholerae O139. Carbohydr Res. 2006 Jul 3;341(9):1077-80. [3]. David J Claffey, et al. Glycosylation of l,4:3,6-dianhydro-D-glucitol (isosorbide). Carbohydr Res. 2004 Oct 4;339(14):2433-40.

6-O-(Triphenylmethyl)-α-D-glucopyranose, 6-O-TRIPHENYLMETHYL-ALPHA-D-GLUCOPYRANOSE, C25H26O6 CAS NO.54325-28-9 MW422.48, Assay (NMR) 98%, bp595.6±50.0 °C at 760 mmHg
-α-D-glucopyranose.png)
(3S,4R)-Methyl 3-(4-chlorobenzoyl)-6-(4-chlorophenyl)-3,4,5-trihydroxy-6-oxohexanoate, C20H18Cl2O6 CAS NO.99886-53-0 MW425.26, Assay (NMR) 97%, bp521.0±50.0 °C at 760 mmHg, Storage: 2-8°C
-Methyl 3-(4-chlorobenzoyl)-6-(4-chlorophenyl)-3,4,5-trihydroxy-6-oxohexanoate.png)
(2R,3S,4S,5R,6R)-2-(((tert-Butyldiphenylsilyl)oxy)methyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol, C23H32O6Si CAS NO.143249-53-0 MW432.59, Assay (NMR) 98%, bp522.9±50.0 °C at 760 mmHg,
-2-(((tert-Butyldiphenylsilyl)oxy)methyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol.png)
Methyl 2,3,5-tri-O-benzyl-D-ribofuranoside, C27H30O5 CAS NO.64363-77-5 MW434.52, Assay (NMR) 98%, bp235 °C at 0.3 mmHg, [1]. Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr Pharm Des. 2012;18(23):3373-88.
(3S,4R,5R,6S)-3,4,5-Tris(benzyloxy)-6-methyltetrahydro-2H-pyran-2-ol, 6-Methyl-3,4,5-tris(phenylmethoxy)oxan-2-ol, C27H30O5 CAS NO.60431-34-7 MW434.53, form SOlid, color White to Off-White, Assay (NMR) 98%, mp89-91 °C, bp507.58 °C at 760 mmHg, Solubility: Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly), Storage: -20℃, An intermediate used for the preparation of selectin blockers and other oligosaccharides, [1]. Susaki, H., et al.: Chem. Pharm. Bull., 42, 1917 (1994), [2]. Kiyoi, T., et al.: Bioorg. Med. Chem. Lett., 8, 2845 (1998),
-3,4,5-Tris(benzyloxy)-6-methyltetrahydro-2H-pyran-2-ol.png)
4-Methylphenyl tetra-O-acetyl-β-D-galactopyranoside, C21H26O10 CAS NO.3520-64-7 MW438.43, Assay _NMR) 98%,

Potassium (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl)oxy)tetrahydro-2H-pyran-2-carboxylate, 2-Oxo-4-(trifluoroMethyl)-2H-1-benzopyran-7-yl β-D-Glucopyranosiduronic Acid MonopotassiuM Salt, C16H12F3KO9 CAS NO.143547-78-8 MW444.36, form SOlid, color White to Off-White, Assay (NMR) 98%, mp>180 °C, Solubility: Methanol (Slightly), Water (Slightly), A glucuronide metabolite of 4-Trifluoromethylumbelliferyl.
-3,4,5-trihydroxy-6-((2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl)oxy)tetrahydro-2H-pyran-2-carboxylate.png)
Trifolirhizin, (−)-Maackiain 3-O-glucoside, C22H22O10 CAS NO.6807-83-6 MW446.40, form solid, Assay ≥95% (LC/MS-ELSD), storage temp. −20°C, application(s) metabolomics vitamins, nutraceuticals, and natural products, Natural product derived from plant source.

TLR4-IN-C34-C2-COOH, C19H29NO11 CAS NO.1159408-54-4 MW447.43, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.91%, bp634.1±55.0 °C at 760 mmHg, Storage: Storage temp. 2-8°C, [1]. Neal MD, et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS One. 2013;8(6):e65779. Published 2013 Jun 12.

Orientin, 2-(3,4-Dihydroxyphenyl)-8-β-Dglucopyranosyl-5,7-dihydroxy-4H-1-benzopyran-4-one, 8-β-D-Glucopyranosyl3',4',5,7-tetrahydroxyflavone, Luteolin 8-Cglucoside, Luteolin 8-C-β-D-glucopyranoside, Luteolin 8-C-β-glucopyranoside, Luteolin-8-glucoside, Lutexin, C21H20O11 CAS NO.28608-75-5 MW448.38, form Solid, color Pale Yellow to Yellow, NMR Conforms to Structure, MS Conforms to Structure, Assay (HPLC) 99.42% (215nm), Solubility: Aqueous Base (Slightly), DMSO (Slightly, Heated), Pyridine (Very Slightly), StorageCondition: -20°C, Orientin is a flavone, a chemical flavonoid compound. Orientin have been investigated for its anti-oxidant, antihypertensive and antihyperlipidemic effects. [1]. Yao, H., et al.: Food Chem., 135, 2802 (2012); [2]. Gordon, A., et al.: Food Chem., 133, 256 (2012); [3]. Cermak, R., et al.: Expert. Opin. Drug. Metab. Toxicol., 4, 17 (2008);

X-GlcNAc, 5-Bromo-4-chloro-3-indolyl N-acetyl-β-D-glucosaminide, C16H18BrClN2O6 CAS NO.4264-82-8 MW449.68, Assay (NMR) 98%, mp240 °C, bp777.5±60.0 °C at 760 mmHg, 5-Bromo-4-chloro-3-indolyl N-acetyl-β-D-glucaminide is a histochemical substrate for N-Acetylglucosaminidase. It releases an insoluble blue chromogen upon enzymatic action.

(2R,3S)-5-Chloro-2-(((4-nitrobenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-nitrobenzoate, 1-Chloro-3,5-diparanitrobenzoyl-2-deoxy-D-ribose, C19H15ClN2O9 CAS NO.51841-98-6 MW450.78, Assay (NMR) 95%, mp107-109 °C, bp646.0±55.0 °C at 760 mmHg
-5-Chloro-2-(((4-nitrobenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-nitrobenzoate.png)
2-deoxy-2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)-β-D-Glucopyranosyl azide 3,4,6-triacetate, 3,4,6-Tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl Azide, C20H20N4O9 CAS NO.102816-24-0 MW460.40, Assay (NMR) 98%, mp135-137 °C, 2-Deoxy-2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)-β-D-glucopyranosyl azide 3,4,6-Triacetate is used to synthesize core fucosylated N-glycans and asparagine-linked glycan chains, stereoselectively. [1]. Szilagyi, Laszlo Gyoergydeak, Zoltan Carbohydrate Research, 1985, vol. 143, p. 21-42. [2]. Ott, D., et al.: Eur. J. Org. Chem., 26, 5054 (2012); [3]. Dan, A., et al.: Chem. Eur. J., 4, 2182 (1998)
-β-D-Glucopyranosyl azide 3,4,6-triacetate.png)
CAS NO.17912-87-7

Estrone beta-D-glucuronide sodium salt, C24H29NaO8 CAS NO.15087-01-1 MW468.48, form Solid, color White to Pale Brown, NMR Conforms to Structure, MS Conforms to Structure, Assay (HPLC 205nm) 97%, Specific Rotation Report Result +28.1° (c = 0.5, Methanol), mp286-290 °C, Water 2.9% by Karl Fischer, Solubility: DMSO (Slightly), Methanol(Slightly, Heated, Sonicated),Water (Slightly), Storage: 2-8°C, sealed storage, away from moisture, A metabolite of 17b -Estradiol, [1]. Qinfang Shen, et al. Adenine nucleotide translocator cooperates with core cell death machinery to promote apoptosis in Caenorhabditis elegans. Mol Cell Biol. 2009 Jul;29(14):3881-93. [2]. Cekan, S., et al.: J. Steroid Biochem., 27, 95 (1989), [3]. Martin, R., et al.: Clin. Chem., 46, 100 (2000), [4]. Blackwell, L., et al.: Steroids, 68, 465 (2003)
n-Nonyl β-D-maltopyranoside, C21H40O11 CAS NO.106402-05-5 MW468.54, Assay (NMR) 98%, Storage: 2-8°C, [1]. M Gabriella Santonicola, et al. Binding of alkyl polyglucoside surfactants to bacteriorhodopsin and its relation to protein stability. Biophys J. 2008 May 1;94(9):3647-58.
Phlorizin (dihydrate), Floridzin (dihydrate), C21H28O12 CAS NO.7061-54-3 MW472.44, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structureMS: Consistent with structurePurity (NMR): ≥98.0%, mp113-114°C, bp770°C at 760 mmHg, Flash point 270.7°C, Storage: Storage temp. 2-8°C, Phloridzin is a glycoside that exists in the leaves and bark of apple trees. It is also present in the skin and flesh of apple fruits.hein is a compound belonging to the class of dihydrochalcones. Rhein is broken down into rhein and glucose by lactase in the epithelial cells the small intestine. Rhein is an antidiabetic drug with the same bioavailability as rhein. [1]. Eric Dale Buras, et al. Proinsulin-producing, hyperglycemia-induced adipose tissue macrophages underlie insulin resistance in high fat-fed diabetic mice. FASEB J. 2015 Aug;29(8):3537-48.
.png)
2,3,4-Tri-O-acetyl-α-D-glucuronide methyl ester trichloroacetimidate, 2,3,4-Tri-O-acetyl-alpha-D-glucuronic Acid Methyl Ester Trichloroacetimidate, C15H18Cl3NO10 CAS NO.92420-89-8 MW478.66, form Solid, color White to light yellow to beige, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, OR[α](C=1.31 g/100ml CHCL3): 95.2°, mp103-105 °C, bp442.1±55.0 °C at 760 mmHg, Solubility: Chloroform (Slightly), DMSO(Slightly), Ethyl Acetate(Slightly), Methanol (Slightly), Storage: -20°C, stored under nitrogen, Intermediate for the synthesis of various glucuronide derivatives, [1]. Keinan et al.: J. Org. Chem. 49, 4988 (1984); [2]. Werschkun, B., et al.: J. Carbohyd. Chem., 18, 629 (1999), [3]. Tietze, L.F., et al.: J. Med. Chem., 52, 537 (2009),

β-D-Glucopyranosiduronic acid,2-formyl-4-nitrophenyl,methyl ester,2,3,4-triacetate, C20H21NO13 CAS NO.148579-83-3 MW483.38, Assay (NMR) 98%, bp583.6±50.0 °C at 760 mmHg

O-[2-(Acetamido)-2-deoxy-3-O-β-D-galactopyranosyl-α-D-galactopyranosyl]-L-threonine, Gal beta(1-3)GalNAc-alpha-Thr, C18H32N2O13 CAS NO.60280-58-2 MW484.45, Assay (NMR) 96%, mp >170 °C
-2-deoxy-3-O-β-D-galactopyranosyl-α-D-galactopyranosyl]-L-threonine.png)
((2R,3R,4S,5S,6S)-6-(Allyloxy)-3,4,5-tris(benzyloxy)tetrahydro-2H-pyran-2-yl)methanol, C30H34O6 CAS NO.93451-42-4 MW490.59, Assay (NMR) 98%,
-6-(Allyloxy)-3,4,5-tris(benzyloxy)tetrahydro-2H-pyran-2-yl)methanol.png)
Benzyl 2-acetamido-3,6-di-O-benzyl-2-Deoxy-alpha-D-glucopyranoside, N-((2S,3R,4R,5S,6R)-2,4-Bis(benzyloxy)-6-((benzyloxy)methyl)-5-hydroxytetrahydro-2H-pyran-3-yl)acetamide, C29H33NO6 CAS NO.55287-49-5 MW491.58, Assay (NMR) 98%, mp140-141 °C, bp694.6±55.0 °C at 760 mmHg, [1]. Carbohydrate research, vol. 94, #2 p. 143-163, [2]. Petit, Jean-michel Jacquinet, Jean-Claude Sinay, Pierre Carbohydrate Research, 1980, vol. 82. p. 130-134, [3]. Paulsen, Hans Kolar, Cenek Chemische Berichte, 1981, vol. 114, # 1 p. 306-321, [4]. Canadian Journal of Chemistry, vol. 70, # 10 p. 2607-2617, [5]. Bulletin of the Academy of Sciences of the US SR, Division of Chemical Science (English Translation), vol. 35 # 3 p. 614-617 Izvestiya Akademii Nauk S S SR, Seriya Khimicheskaya, # 3 p. 671-675

2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl trichloroacetimidate, C16H20Cl3NO10 CAS NO.92052-29-4 MW492.69, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥98.0%, mp154.0-160.0 °C, bp463.3±55.0°C at 760 mmHg, Storage: Storage temp. -20°C

CAS NO.1852-49-9

(2R,3S,4S,5R,6S)-2-(Acetoxymethyl)-6-(4-(hydroxymethyl)-2-nitrophenoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate, C21H25NO13 CAS NO.290298-12-3 MW499.42, Assay (NMR) 97%, Storage: 2-8°C
-2-(Acetoxymethyl)-6-(4-(hydroxymethyl)-2-nitrophenoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate.png)
Delphinidin 3-β-D-Glucoside, C21H21O12Cl CAS NO.6906-38-3 MW500.84, A metabolite of Delphinidin. A constituent of blue honeysuckle as to novel source for phenolic antioxidants. Antimicrobial. [1]. Christensen, G., et al.: J. Clin. Microbiol., 22, 996 (1985),

2,3,4,6-Tetra-O-pivaloyl-β-D-galactopyranosylamine, 2,3,4,6-Tetra-O-pivaloyl-beta-D-galactopyranosylamine, C26H45NO9 CAS NO.108342-87-6 MW515.64, Assay (NMR) 97%, mp92-95°C, bp528.7ºC at 760 mmHg, Flash point 75.9ºC, [1]. Pleuss, Norbert Kunz, Horst Synthesis, 2005, # 1 art. no. T07304SS, p. 122-130, [2]. Knauer, Stefan Weymann, Markus Kunz, Horst Zeitschrift fur Naturforschung - Section B Journal of Chemical Sciences, 2009, vol. 64, # 11-12 p. 1639-1652
CAS NO.64461-95-6

(3S,4R,5S,6S)-6-(4-chloro-3-(4-ethoxybenzyl)phenyl)tetrahydro-2H-pyran-2,3,4,5-tetrayl tetraacetate, C28H31ClO10 CAS NO.1018898-84-4 MW562.99, Assay (NMR) 98%, Storage: 2-8°C
-6-(4-chloro-3-(4-ethoxybenzyl)phenyl)tetrahydro-2H-pyran-2,3,4,5-tetrayl tetraacetate.png)
Vitexin-2-O-Rhamnoside, 4H-1-Benzopyran-4-one, 8-[2-O-(6-deoxy-αL-mannopyranosyl)-β-D-glucopyranosyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-, C27H30O14 CAS NO.64820-99-1 MW578.52, form Solid, color Light Yellow to Yellow, NMR Conforms to Structure, MS Conforms to Structure, Assay (HPLC 215nm) 99.15%, Solubility: DMSO (Slightly), Water(Slightly), Vitexin-2-O-Rhamnoside is a phenolic glycoside with potential anti-proliferative activity. Anti-obesity agent, adipocyte differentiation inhibitor. [1]. Wei, W. et al.: J. Pharm. Pharmacol., 66, 988 (2014); [2]. Gonzalez-Castejon, M. et al.: Phytother. Res., 28, 745 (2014);

CAS NO.15397-15-6

CAS NO.118977-26-7

CAS NO.81058-26-6

CAS NO.956107-32-7

(3R,4S,5R,6R)-6-(acetoxymethyl)-2-(3-((5-(2-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triyl triacetate, C33H35FO10S MW642.69, Assay (NMR) 98%
-6-(acetoxymethyl)-2-(3-((5-(2-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triyl triacetate.png)
CAS NO.842133-80-6

(3R,3'R,4S,4'S,5S,5'S,6R,6'R)-2,2'-(([2,2'-bithiophene]-5,5'-diylbis(methylene))bis(4-methyl-3,1-phenylene))bis(6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol), C38H46O12S2 MW758.89, Assay (NMR) 95%
-2,2'-(([2,2'-bithiophene]-5,5'-diylbis(methylene))bis(4-methyl-3,1-phenylene))bis(6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol).png)
CAS NO.57817-89-7

CAS NO.37271-16-2

HM-140_3 impurity A, C54H62O20S2 MW1095.19, Assay (NMR) 98%

CAS NO.121584-52-9

CAS NO.93460-33-4

CAS NO.10297-09-3

CAS NO.7218-43-1

CAS NO.15231-41-1

CAS NO.929-59-9

CAS NO.216700-85-5

CAS NO.86520-52-7

CAS NO.170304-76-4

CAS NO.54149-17-6

CAS NO.57078-98-5

CAS NO.151978-50-6

CAS NO.2127875-65-2

CAS NO.85909-08-6

CAS NO.132178-37-1

CAS NO.2050-25-1

CAS NO.117235-10-6

CAS NO.533-48-2

CAS NO.106627-54-7

CAS NO.142929-49-5

CAS NO.86770-67-4

CAS NO.784105-33-5

CAS NO.143-24-8

CAS NO.1380500-88-8

CAS NO.6404-29-1

CAS NO.5414-19-7

CAS NO.172531-37-2

CAS NO.1948273-09-3

CAS NO.60444-78-2

CAS NO.153086-78-3

CAS NO.55750-62-4

CAS NO.524030-00-0

CAS NO.201467-81-4

CAS NO.252881-74-6

CAS NO.186020-66-6

CAS NO.31127-85-2

CAS NO.1835705-53-7

CAS NO.2381196-79-6

CAS NO.1119249-30-7

CAS NO.25322-68-3

Tos-PEG3,2-[2-(2-hydroxyethoxy)ethoxy]ethyl 4-methylbenzenesulfonate, C13H20O6S CAS NO.77544-68-4 MW304.36, form Solid-Liquid Mixture, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 97.39%, Storage: Storage temp. 2-8°C
CAS NO.1353016-70-2

CAS NO.55750-63-5

CAS NO.731862-92-3

SPDP, SPDP Crosslinker, Py-ds-Prp-OSu, C12H12N2O4S2 CAS NO.68181-17-9 MW312.36, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.70%, Storage: -20°C, protect from light, stored under nitrogen
3-(3-Propyl-1,2,4-oxadiazol-5-yl)-N-(1H-1,2,4-triazol-5-yl)propanamide, C10H14N6O2 CAS NO.1087922-12-0 MW250.26, Assay 98%
-N-(1H-1,2,4-triazol-5-yl)propanamide.png)
CAS NO.77472-98-1

Propargyl-PEG6-NH2, C15H29NO6 CAS NO.1198080-04-4 MW319.39, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Purity (NMR): ≥98.0%, Storage: Storage temp. -20°C
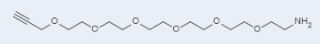
CAS NO.2376724-97-7

CAS NO.194920-62-2

CAS NO.1425973-14-3

CAS NO.79642-50-5

CAS NO.115088-06-7

CAS NO.756525-98-1

CAS NO.64987-85-5

CAS NO.439114-13-3

CAS NO.260367-12-2

CAS NO.2358775-67-2

CAS NO.77855-76-6

CAS NO.200335-40-6

CAS NO.192132-77-7

CAS NO.126519-80-0

CAS NO.68528-80-3

CAS NO.1799506-30-1

CAS NO.138529-46-1

CAS NO.159857-79-1

CAS NO.869718-81-0

CAS NO.166108-71-0

CAS NO.1807539-10-1

CAS NO.2378261-81-3

CAS NO.155130-15-7


CAS NO.1333154-77-0

CAS NO.872679-70-4

CAS NO.1353016-71-3

CAS NO.57757-57-0

CAS NO.2381196-81-0

CAS NO.359860-27-8

CAS NO.211859-73-3

CAS NO.139338-72-0

CAS NO.217817-01-1

CAS NO.86259-89-4

CAS NO.92921-24-9

CAS NO.187794-49-6

CAS NO.70539-42-3

CAS NO.19249-03-7

CAS NO.169751-73-9

CAS NO.663171-32-2

CAS NO.206265-98-7

CAS NO.1599440-15-9

CAS NO.437655-95-3

CAS NO.2378261-80-2

CAS NO.557756-85-1

CAS NO.1334177-84-2

Azido-PEG5-triethoxysilane, C22H46N4O9Si MW538.71, Assay (NMR) 98%
.png)
Boc-Gly-Gly-Phe-Gly-OH (TFA), C22H29F3N4O9 CAS NO.2450273-39-7 MW550.48, Assay (NMR): 98%, Storage: polypeptide, sealed storage, away from moisture
.png)
2-((Azido-PEG8-carbamoyl)methoxy)acetic acid, CAS NO.846549-37-9 C22H42N4O12 MW554.59, form Liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (NMR): ≥98.0%, Storage: Storage temp. -20°C
methoxy)acetic acid.png)
Fmoc-Gly-Gly-D-Phe-OtBu, C32H35N3O6 CAS NO.236426-37-2 MW557.64, Assay (NMR) 98%, bp806.7±65.0 °C at 760 mmHg, Storage: 2-8°C, stored under nitrogen, [1]. Bak A, et al. Synthesis and evaluation of the physicochemical properties of esterase-sensitive cyclic prodrugs of opioid peptides using an (acyloxy)alkoxy linker. J Pept Res. 1999 Apr;53(4):393-402.

DBCO-CONH-S-S-NHS ester,C28H27N3O6S2 CAS NO.1435934-53-4 MW565.66, Assay 98%, Storage: -20°C, protect from light, stored under nitrogen, [1]. Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017 May;16(5):315-337.

MC-Val-Cit-PAB, C28H40N6O7 CAS NO.159857-80-4 MW572.65, form Solid, color White to yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 99.29%, bp931.6±65.0 °C at 760 mmHg, Storage: 2-8°C, protect from light, stored under nitrogen, MC-Val-Cit-PAB intermediate used as a reagent for the preparation of antibody-drug conjugates, a compound that selectively delivers cytotic drugs to tumor-associated antigens. [1]. Anti-cd70 antibody-drug conjugates and their use for the treatment of cancer and immune disorders

Bis-PEG6-NHS ester, C24H36N2O14 CAS NO.1526718-98-8 MW576.55, form Viscous liquid, color Colorless to light yellow, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, Storage: -20°C, sealed storage, away from moisture, [1]. John L. Magna, et al. Highly potent multimeric e-selectin antagonists. WO2018068010A1.
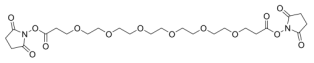
Biotin-PEG4-NHS ester,5-[(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl]-N-{15-[(2,5-dioxo-1-pyrrolidinyl)oxy]-15-oxo-3,6,9,12-tetraoxapentadec-1-yl}pentanamide, C25H40N4O10S CAS NO.459426-22-3 MW588.67, form Solid, color White to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 97.29%, Storage: -20°C, sealed storage, away from moisture and light, Pegylated reagents for biotin labeling of antibodies and proteins,[1]. Gadd MS, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017 May;13(5):514-521.
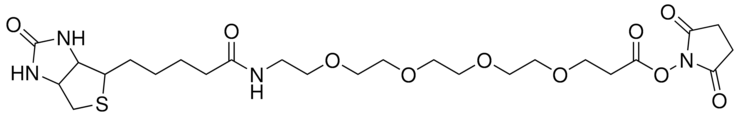
Biotin-PEG4-methyltetrazine, C27H39N7O6S CAS NO.1835759-81-3 MW589.71, form SOlid, color Purple to purplish red, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (LCMS): 98.14%, Storage: 2-8°C, sealed storage, away from moisture, [1]. An S, et al. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 2018 Oct;36:553-562

Mc-Val-Cit-PAB-Cl, C28H39ClN6O6 CAS NO.1639351-92-0 MW591.10, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥95.0%, 915.4±65.0 °C at 760 mmHg, Storage: -20°C, stored under nitrogen, Mc-Val-Cit-PAB-Cl is a cleavable ADC linker for coupling MMAE and antibodies to form antibody-MC-vcMMAE. [1]. Staben LR, et al. Stabilizing a Tubulysin Antibody-Drug Conjugate To Enable Activity Against Multidrug-Resistant Tumors. ACS Med Chem Lett. 2017 Sep 5;8(10):1037-1041.
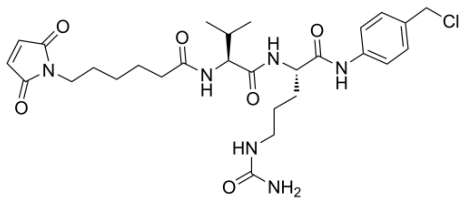
Mal-amido-PEG8-C2-acid, Carboxy-PEG-maleimide, Carboxy-PEG8-maleimide, MAL-PEG400-acid, MAL-PEG8-COOH, MAL-PEG8-acid, C26H44N2O13 CAS NO.1334177-86-4 MW592.63, form solid or viscous liquid, color Colorless to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥97.0%, bp747.9±60.0 °C at 760 mmHg, [1]. Paul Joseph Mark JACKSON, et al. PDD Compounds. Patent. US2018339985.

Phe-Lys(Fmoc)-PAB, C37H40N4O5 CAS NO.2149584-03-0 MW620.74, Phe-Lys(Fmoc)-PAB is a peptide precursor linker. [1]. Abu Ajaj K, et al. Development of protein-binding bifunctional linkers for a new generation of dual-actingprodrugs. Bioconjug Chem. 2009 Feb;20(2):390-6.
-PAB.png)
DPPC, 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine, PC, (7R)-4-Hydroxy-N,N,N-trimethyl-10-oxo-7-[(1-oxohexadecyl)oxy]- 3,5,9-trioxa-4-phosphapentacosan-1-aminium 4-oxide, inner salt, 1,2-Dihexadecanoyl-sn-glycero-3-phosphocholine, 1,2-Dihexadecanoyl-sn-glycero-3-phosphorylcholine, 1,2-Dipalmitoyl-sn-glycero-3-phosphorylcholine, 1,2-Dipalmitoyl-L-lecithin, L-α-Phosphatidylcholine, dipalmitoyl, L-β,γ-Dipalmitoyl-α-lecithin, C40H80NO8P CAS NO.63-89-8 MW734.04, form powder, color White to off-white, 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 99.56%, Optical Rotation: 6.7°(C=0.01g/ml CHCL3), mp229-229.5 °C, Storage: -20°C, sealed storage, away from moisture, [1]. Akiko Uemura, et al. Induction of immune responses against glycosphingolipid antigens: comparison of antibody responses in mice immunized with antigen associated with liposomes prepared from various phospholipids. J Vet Med Sci. 2005 Dec;67(12):1197-201. [2]. Miller AD. Delivery of RNAi therapeutics: work in progress. Expert Rev Med Devices. 2013 Nov;10(6):781-811. [3]. Agustín Mangiarotti, et al. Phase coexistence in films composed of DLPC and DPPC: a comparison between different model membrane systems. Biochim Biophys Acta. 2014 Jul;1838(7):1823-31. [4]. David S Wishart, et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 2009 Jan;37(Database issue):D603-10. [5]. Fabiana Rached, et al. Defective functionality of HDL particles in familial apoA-I deficiency: relevance of alterations in HDL lipidome and proteome. J Lipid Res. 2014 Dec;55(12):2509-20.
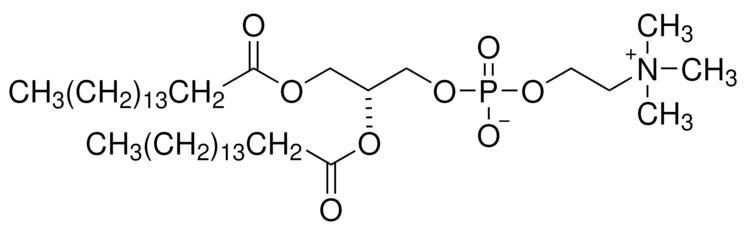
Mc-Val-Cit-PABC-PNP, L-Ornithinamide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aminocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]methyl]phenyl]-, C35H43N7O11 CAS NO.159857-81-5 MW737.76, form powder, color Off-white to light yellow, 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (HPLC): 98.45%, bp1034.5±65.0°C at 760 mmHg, Storage: 2-8°C, protect from light, stored under nitrogen, Mc-Val-Cit-PABC-PNP is a bifunctional cathepsin cleavable linker used in the construction of antibody drug conjugates (ADC). The maleimide functionality can be used for bioconjugation to thiols in monoclonal antibodies while the activated carbonate esters can be used for construction of carbamate linkages with amine containing payloads. [1]. Che-Leung Law et al. Anti-cd70 antibody-drug conjugates and their use for the treatment of cancer and immune disorders.Patent WO2005081711A2. [2]. WO2007/8603 A1, Page/Page Column 137 166 167.
1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine, C41H82NO8P CAS NO.1069-79-0 MW748.07, form Solid, color White to off-white, 1 H NMR Spectrum: Consistent with structure, Assay (HPLC): 98.07%, mp172-173°C, Solubility: Chloroform (Slightly, Heated, Sonicated), 20 mg/ml in Chloroform/Methanol 3:1,Colorless,clear, Storage: Storage temp. 2-8°C, 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine is used in the generation of micelles and liposomes. [1]. McCarley RL, et al. Redox-responsive delivery systems. Annu Rev Anal Chem (Palo Alto Calif). 2012;5:391-411. [2]. Jun Chen, et al. Characterization of 9-nitrocamptothecin-in-cyclodextrin-in-liposomes modified with transferrin for the treating of tumor. Int J Pharm. 2015 Jul 25;490(1-2):219-28. [3]. Kevin J Kauffman, et al. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015 Nov 11;15(11):7300-6. [4]. Michelle Fraga, et al. Influence of phospholipid composition on cationic emulsions/DNA complexes: physicochemical properties, cytotoxicity, and transfection on Hep G2 cells. Int J Nanomedicine. 2011:6:2213-20. [5]. Naila El Kechai, et al. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int J Pharm. 2015 Jun 20;487(1-2):187-96. [6]. HГёyrup, P., et al.: Biochim. Biophys. Acta, 1515, 133 (2001); [7]. Heberle, F., et al.: Cold Spring Harb Perspect Biol., 3, 1 (2011)

Cyanine 5, SE (Technical Grade), 2-[5-[1-[6-[(2,5-Dioxo-1-pyrrolidinyl)oxy]-6- oxohexyl]-1,3-dihydro-3,3-dimethyl-5-sulfo2H-indol-2-ylidene]-1,3-pentadien-1-yl]-1- ethyl-3,3-dimethyl-5-sulfo-3H-indolium, Inner Salt, Cy5 NHS Ester, C37H43N3O10S2 CAS NO.146368-14-1 MW753.881, form Solid, color Dark Purple to Very Dark Purple, MS Conforms to Structure, UV Absorbance 193 nm, 259 nm, 332 nm, 647 nm, NMR Conforms to Structure, Storage: 4°C, Cyanine 5, SE is a useful research chemical. Cyanine 5 is a cyanine dye labeling reagent for biochemistry. [1]. Mujumdar, R., et al.: Bioconjugate Chem., 4, 105 (1993);
Fmoc-Val-Cit-PAB-PNP, C40H42N6O10 CAS NO.863971-53-3 MW766.80, form Solid, color White to yellow (Solid), 1 H NMR Spectrum: Consistent with structure, MS: Consistent with structure, Assay (HPLC): 98.69%, mp189 °C, bp1024.0±65.0 °C at 760 mmHg, Storage: -20°C, stored under nitrogen, FMoc-Val-Cit-PAB-PNP is an organic intermediate that can be used to prepare peptide conjugates. [1]. Dorywalska M, et al. Effect of attachment site on stability of cleavable antibody drug conjugates. Bioconjug Chem. 2015 Apr 15;26(4):650-9. [2]. Dubowchik GM, et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002 Jul-Aug;13(4):855-69. [3]. Yoneda Y, et al. A cell-penetrating peptidic GRP78 ligand for tumor cell-specific prodrug therapy. Bioorg Med Chem Lett. 2008 Mar 1;18(5):1632-6.

Mal-PEG2-VCP-NB, C36H45N7O13 CAS NO.2395887-69-9 MW783.78, Assay (NMR) 95%, Storage: 2-8°C, stored under nitrogen
Biotin-PEG4-amino-t-Bu-DADPS-C6-azide, C43H67N7O9SSi CAS NO.1260247-50-4 MW886.18, form Solid, color Off-white to light yellow, Assay: ≥95.0%, Storage: Storage temp. -20°C, Biotin-PEG4-amino-t-Bu-DADPS-C6-azide is a PROTAC linker belonging to the PEG class. It can be used for the synthesis of PROTAC molecules. *This product is unstable in solution state, and it is recommended to use it immediately after preparation. [1]. An S, et al. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 2018 Oct;36:553-562

CAS NO.122116-12-5

CAS NO.98627-69-1

CAS NO.79598-48-4

CAS NO.51-85-4

CAS NO.1155811-37-2

CAS NO.356066-46-1

CAS NO.791028-27-8

CAS NO.1334286-77-9

CAS NO.208827-90-1

CAS NO.882518-90-3

CAS NO.209542-49-4

CAS NO.1027371-75-0

CAS NO.19364-66-0

CAS NO.85030-56-4

CAS NO.106492-60-8

CAS NO.93206-09-8

CAS NO.112724-27-3

CAS NO.1380500-92-4

CAS NO.4671-77-6

CAS NO.134179-38-7

CAS NO.660843-22-1

CAS NO.1013921-36-2

CAS NO.1260092-44-1

CAS NO.67319-28-2

CAS NO.64987-82-2

CAS NO.55489-58-2

CAS NO.869310-84-9

CAS NO.1101668-39-6

CAS NO.1056024-94-2

CAS NO.23778-52-1

CAS NO.159428-42-9

CAS NO.50586-80-6

CAS NO.81836-43-3

CAS NO.39028-27-8

CAS NO.86259-87-2

CAS NO.23601-40-3

CAS NO.252881-73-5

CAS NO.1245823-51-1

CAS NO.134179-40-1

CAS NO.77855-75-5

CAS NO.62921-74-8

CAS NO.1347750-72-4

CAS NO.57671-28-0

CAS NO.622405-78-1

CAS NO.77544-60-6

CAS NO.105589-77-3

CAS NO.98627-22-6

CAS NO.905954-28-1

CAS NO.400775-35-1

CAS NO.62921-76-0

CAS NO.874208-91-0

CAS NO.24342-68-5

CAS NO.367927-39-7

CAS NO.119189-70-7

CAS NO.25990-96-9

Cbz-NH-PEG4-C2-acid, C19H29NO8 CAS NO.756526-00-8 MW399.44, form Liquid, color Colorless to light yellow (Liquid), 1 H NMR Spectrum: Consistent with structure, LCMS: Consistent with structure, Assay (NMR): ≥95.0%, Storage: Storage temp. 2-8°C

CAS NO.80755-67-5

CAS NO.120550-35-8

CAS NO.3386-18-3

CAS NO.955094-26-5

CAS NO.158913-22-5

CAS NO.6048-68-6

CAS NO.756526-04-2

CAS NO.1092554-87-4

CAS NO.252881-76-8

CAS NO.882847-13-4

CAS NO.2086689-02-1

CAS NO.874208-92-1

CAS NO.1028089-05-5

CAS NO.756526-06-4

CAS NO.928292-69-7

CAS NO.756525-90-3

CAS NO.756525-99-2

CAS NO.854601-60-8

CAS NO.882847-32-7

CAS NO.1314378-18-1

CAS NO.1352827-47-4

CAS NO.1314378-14-7

CAS NO.1036204-61-1

CAS NO.778596-26-2

CAS NO.1402080-11-8

CAS NO.1189112-05-7

CAS NO.1225057-68-0

CAS NO.1345337-28-1

CAS NO.756526-02-0

CAS NO.28821-35-4

CAS NO.756525-93-6

CAS NO.870487-08-4

CAS NO.2101200-09-1

CAS NO.173308-19-5

CAS NO.1211568-27-2

CAS NO.381722-48-1

CAS NO.1267337-47-2

CAS NO.2304513-76-4

CAS NO.1448297-52-6

CAS NO.137076-54-1

CAS NO.35665-38-4

CAS NO.23243-68-7

CAS NO.843666-27-3

CAS NO.1076199-21-7

CAS NO.142356-33-0

CAS NO.875770-34-6

CAS NO.1957236-21-3


TRIS, 2-Amino-2-(hydroxymethyl)-1,3-propanediol, THAM, Tris base, Tris(hydroxymethyl)aminomethane, Trometamol, NH2C(CH2OH)3 CAS No.77-86-1 MW121.14 EC No.201-064-4 Beilstein No.741883, Assay 99.9%(Titration with H2SO4 (Anhydrous)), form crystalline solid, color white, pH10.2-10.6 (20 °C, 6 g/L in H2O), useful pH range7-9, pKa (25 °C)8.1, bp219-220 °C/10 mmHg (lit.) 219-220 °C/13.3 hPa, mp167-172 °C (lit.), solubility 678 g/L, density 1.35 g/cm3 at 23 °C, application(s) ophthalmics pharmaceutical, storage temp.no temp limit, Tris(hydroxymethyl)aminomethane (Trometamol) is a commonly used buffer component in biomolecule downstream chromatography steps as well as final liquid formulation. General description Tris(hydroxymethyl)aminomethane, commonly known as Trometamol, Tris base, serves a pivotal role in diverse research applications as a biological buffer. Its optimal pKa of 8.1 makes it a preferred choice for formulating buffers like Tris-acetate-EDTA (TAE) and Tris-borate-EDTA (TBE), ensuring the maintenance of pH within the physiological range (pH 7 - 9), applicable to a wide spectrum of living organisms. Despite its utility, researchers need to exercise caution in protein studies, as Tris has the potential to interfere with the activity of specific enzymes. Tris base may find application as basimetric standard, independently as a buffer and as a crucial component in mixed buffer formulations, including Tris-EDTA (TE) buffer, TAE buffer, TBE buffer, among others. Its attributes include purity, essential stability, and a relative non-hygroscopic nature, making it a dependable choice in laboratory settings. In these environments, Tris base is indispensable for preparing buffers compatible with biological fluids and serves as a standard pH solution. It facilitates various laboratory procedures such as lactate dehydrogenase assays, in situ hybridization, and protein extraction from cells. The versatility of Tris base extends to cell biology, biochemistry, and protein research contributing significantly to studies involving cell membrane permeability and buffer preparation. Application Tris has been used: as a component of H buffer (cell dissociation buffer), for washing and saturation of wells in double sandwich ELISA immunoenzymatic technique, as an assay buffer for reconstitution of extracted and dried protein samples, to prepare Tris-HCl buffer that is used to stabilize proteins, as a buffer to extract carotenoid from tubers, as a component of sample buffer during protein extraction prior to western blotting, as a component of sample buffer for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), to prepare simulated body fluid (SBF) for calcium phosphate (CaP) resorption assay, as a buffer for polydopamine (PDA) deposition on stainless steel (SS) substrate. Features and Benefits Efficient buffering within the pH range of 7 - 9 with a pKa of 8.1 (25 °C), Tested to confirm low levels of heavy metal, contamination, ensuring suitability for various applications, Can be used in Cell Biology, and Biochemical research.
TRIS-HCL,Tris(hydroxymethyl)aminomethane hydrochloride, TRIS HCl, TRIS hydrochloride, Tris(hydroxymethyl)aminomethane hydrochloride, Tromethane hydrochloride, NH2C(CH2OH)3·HCl CAS No.1185-53-1 MW157.60 Beilstein No.3675235, biological source synthetic, reagent grade, assay ≥99%, form crystalline, storage condition (Tightly closed. Dry), impurities <0.5% water, color white, useful pH range 7.0-9.0, pKa (25 °C)8.1, mp~150.7 °C, solubility water: 1.5 g/mL, clear, colorless, cation traces Fe: ≤5 ppm Pb: ≤5 ppm,storage temp.room temp, General description Tris HCl, serves as a widely utilized acidic buffer with a pKa of 8.08 at 25°C, offering a pH range of 7.0 to 9.0, aligning with the typical pH of living organisms. It is a crucial component in buffer solutions for diverse biological, cell biology, and biochemistry applications. Notably, Tris base and Tris hydrochloride individually lack sufficient buffering capacity, necessitating their combination to create effective buffers within the pH range of 7 to 9. Essential in protein electrophoresis and western blotting techniques, Tris buffers, particularly low ionic strength versions, are employed for protein transfer. They are also commonly used in SDS-PAGE gels, running buffers, and blotting buffers. Additionally, Tris salts, integral to DNA agarose electrophoresis, contribute to buffer preparations like TAE (Tris acetate/EDTA) and TBE (Tris borate/EDTA), playing pivotal roles in biochemistry, protein purification, and electrophoresis applications. Overall, Tris HCl proves to be a versatile and indispensable component, ensuring optimal pH in biological systems for effective biochemical and cell biology research. Features and Benefits Ideal as a Biological buffer for Cell Biology and Biochemical research, Effective Buffering from pH 7-9 (25 °C) with a pKa of 8.1 (25 °C), Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications.
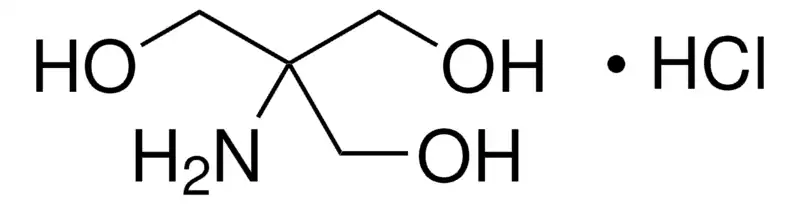
Tris(hydroxymethyl)nitromethane, 2-Hydroxymethyl-2-nitro-1,3-propanediol, NO2C(CH2OH)3 CAS No.126-11-4 MW151.12 EC No.204-769-5 Beilstein No.1768985 UNSPSC Code:12352100, Assay 98%(TLC), 99%(TLC), form solid, color Off-White to Yellow to Yellow Orange, mp160 °C (dec.) (lit.), solubility H2O: soluble 220g/100ml at 20°C, alcohols: freely soluble, benzene: very slightly soluble, hydrocarbons: very slightly soluble, functional group: amine hydroxyl nitro, General description Tris(hydroxymethyl)nitromethane undergoes crosslinking with hexamethylene diisocyanate to form monolithic energetic gels. It is an effective hardener for variety of tannin-based adhesives. Preparation Note 220 g of Tris(hydroxymethyl)nitromethane dissolves in 100 ml of water at 20°C.
nitromethane.png)
Tris(hydroxymethyl)aminomethane acetate, TRIS acetate salt, Tris(hydroxymethyl)aminomethane acetate salt,NH2C(CH2OH)3·CH3COOH CAS No.6850-28-8 MW181.19 EC No.229-939-6 Beilstein No.3702918 UNSPSC Code:12161700, Assay ≥99.0% (titration with NaOH), form crystalline powder, color white, A290 UV absorbance at 290nm (50% aqueous) ≤0.5,pH6.0-7.0 (0.1 M in water, high purity), solubility water: 0.5 M, clear, colorless,Water (by Karl Fischer)≤0.2%, heavy metals as Pb:≤5ppm, Infrared spectrum:conforms to structure, TRIS acetate salt has been used: in the adenosine triphosphate (ATP) assay to neutralize the pH to 7.7 of the oocyte supernatant, in cell wall isolation to remove proteins and membranes from the cell walls of plants, as a component of DamID buffer, lysis buffer for cell sorting and lysing.
aminomethane acetate.png)
BIS-TRIS, 2,2-Bis(hydroxymethyl)-2,2′,2″-nitrilotriethanol, 2-Bis(2-hydroxyethyl)amino-2-(hydroxymethyl)-1,3-propanediol, Bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane, C8H19NO5 CAS No.6976-37-0 MW209.24 EC No.230-237-7 Beilstein No.22052756976-37-0 UNSPSC Code:12161700, suitable for cell culture, suitable for insect cell culture, Assay ≥98.0%, 99%, form crystalline crystals to powder, color White, technique(s) cell culture | insect: suitable cell culture | mammalian: suitable, impurities endotoxin and total aerobic microbial count, tested, useful pH range5.8-7.2 pKa (25 °C)6.5, solubility H2O: 500 mg/mL, clear, colorless to very faintly yellow, application(s) diagnostic assay manufacturing, Application BIS-TRIS can be used to study biological buffers. BIS-TRIS has been used in a study that synthesized ferritin grafted with temperature-responsive poly(N-isopropylacrylamide) (PIPAAm-ferritin) by a coupling reaction using PIPAAm and ferritin for obtaining stimuli-responsive biomaterials. BIS-TRIS has also been used in a study that synthesized and described the photochemical properties of oligo- ortho –azobenzenes. Other Notes Easily compare specifications for BIS-TRIS products with the BIS-TRIS specification table. Effective buffer for the assay of creatine kinasecitation; Effectively protects hemoglobin during freeze-drying Catholyte in IEF of 2-D gel electrophoresis. Effective buffer for the assay of creatine kinase; Effectively protects hemoglobin during freeze-drying; Catholyte in IEF of 2-D gel electrophoresis.
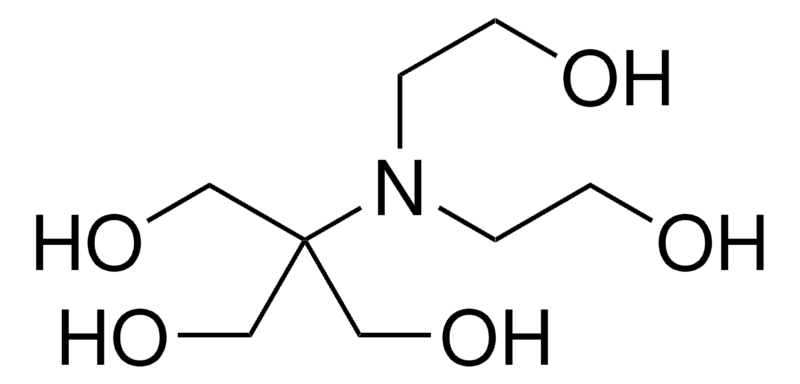
BIS-TRIS Propane, 1,3-Bis[tris(hydroxymethyl)methylamino]propane, 2,2′-(Propane-1,3-diylbis(azanediyl))bis(2-(hydroxymethyl)propane-1,3-diol) CH2[CH2NHC(CH2OH)3]2 CAS No.64431-96-5 MW282.33 EC No.264-899-3 Beilstein No.1786109, Assay ≥99.0% (titration), form crystalline powder, colro white, storage condition dry at room temperature, technique(s) HPLC: suitable, color white, useful pH range6.3-9.5, pKa (25 °C) (1)6.8 (2)9.0, mp164-165 °C (lit.), solubility water: 30 % (w/w), clear, colorless, density 1.179 g/cm3 at 20—25°C 1.189 g/cm3 at 20—25°C, suitability suitable for chromatography suitable for electrophoresis buffers, application(s) diagnostic assay manufacturing general analytical life science and biopharma, General description Bis-Tris Propane buffer, also known as BTP, is a zwitterionic biochemical buffer utilized for pH regulation in various laboratory applications, particularly in molecular biology, biochemistry, and electrophoresis. Its wide buffering range, spanning from pH 6.3 to 9.5, is attributed to its two pKa values of 6.8 (pKa1) and 9.0 (pKa2), which are close. This wide range makes it highly versatile for maintaining stable pH conditions in a variety of experiments. Furthermore, Bis-Tris Propane is a water-soluble buffer substance that plays a crucial role in enhancing the stability and activity of restriction enzymes. Specifically, its buffering capacity is advantageous for experiments that require pH levels as low as 6-7. When compared to Tris buffer, which has a poor buffering capacity below pH 7.5 and exhibits significant pKa fluctuations with temperature changes, Bis-Tris Propane proves to be a superior choice. Additionally, it finds application in conjunction with hydrochloric acid buffer to stabilize farnesyl diphosphate, particularly when isolated from Saccharomyces cerevisiae strains. Notably, it can also serve as a ligand, forming dinuclear hydroxo complexes with Lanthanides(III). Application BIS-TRIS Propane may be used in the following studies: as a buffer to investigate the influence of anions with varying nucleophilic properties on the autoxidation of oxymyoglobin (MbO2). for purification of glucose-binding protein from membranes of Sulfolobus solfataricus. as a buffer during GeO2 mineralisation. as a buffer component in a study pertaining to environmental research. as a buffer component to maintain optimal pH conditions during the purification and preparation of engineered enzymes. Features and Benefits Suitable for Biological and Biochemical Research, Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications Effective Buffering from pH 6.3-9.5 (25 °C) with a pKa of 6.8 and 9.0 (25 °C), Highly soluble in water

Tricine, N-[Tris(hydroxymethyl)methyl]glycine, (HOCH2)3CNHCH2CO2H, CAS No.5704-04-1 MW179.17 EC No.227-193-6 Beilstein No.:1937804 UNSPSC Code:12161700, Assay ≥99% (titration), form crystalline powder, color white, useful pH range 7.4-8.8, pKa (25 °C) 8.1, mp187 °C, solubility water: 0.25 g/mL, clear, colorless, application(s) diagnostic assay manufacturing, General description Tricine is the most frequently used electrophoresis buffer. It is also useful for the resuspension of cell pellets. Tricine functions as a trailing ion and aids the resolution of small proteins at lower acrylamide concentrations than in glycine-sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) systems. It has a lower negative charge than glycine, which helps it migrate faster. Tricine has a high ionic strength that allows increased ion movement and less protein movement. This in turn leads to the separation of low molecular weight proteins in lower percent acrylamide gels. Application Buffer component for separation of low molecular weight peptides. Tricine has been used: to prevent precipitation of salts during autoclaving of Emiliania huxleyi cultures, as a component of buffer A for the homogenization of samples like Caenorhabditis elegans, Drosophila, and plants, as a component of fresh assay buffer to measure serum melatonin by radioimmunoassay.
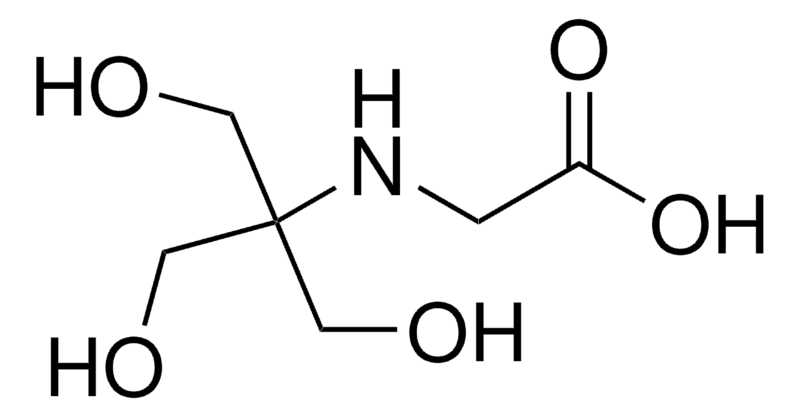
BICINE, N,N-Bis(2-hydroxyethyl)glycine, C6H13NO4 CAS No.150-25-4 MW163.17 Beilstein No.:1769362 EC No.205-755-1 UNSPSC Code:12161700, Assay ≥99% (titration by NaOH)(anhydrous), form powder or crystals, color white, pH3.5-5.0, useful pH range 7.6-9.0, pKa (25 °C)8.3, solubility water: 25 % (w/w), clear, colorless, application(s) diagnostic assay manufacturing, storage temp.room temp, Application Recommended buffer for low temperature biochemical work. Used in the preparation of stable substrate solution for serum guanase determination. Spacer in plasma protein fractionation with BICINE by isotachophoresis.
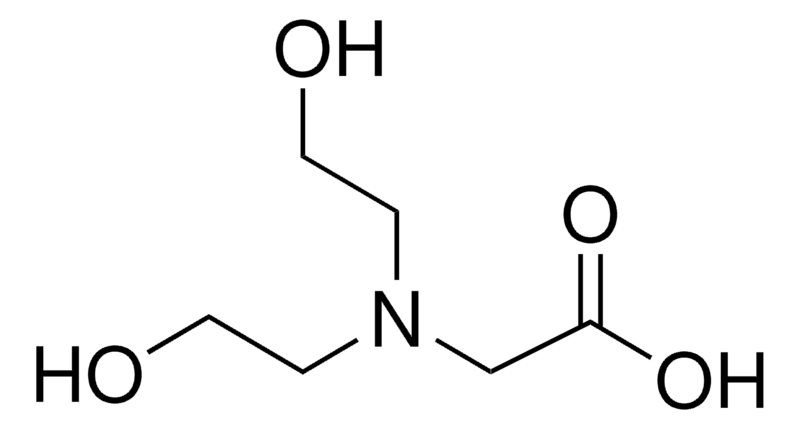
BICINE Na, BICINE-Na, sodium N,N-bis(2-hydroxyethyl)glycinate; sodium,2-[bis(2-hydroxyethyl)amino]acetate;BICINESODIUMSALT;DIETHANOLGLYCINESODIUMSALT; Glycine,N,N’-bis(2-hydroxyethyl)monosodiumsalt;N,N-BIS(2-HYDROXYETHYL)GLYCINESODIUMSALT;N,N-DIHYDROXYETHYLGLYCINE,SODIUMSALT;n,n-bis(2-hydroxyethyl)glycine,monosodiumsalt;n,n-bis(2-hydroxyethyl)-glycinmonosodiumsalt, C6H12NNaO4 CAS No.139-41-3 MW185.15, assay 99%, bp381.42℃[at 101 325 Pa], solubility water:909g/L at 20℃,density 1.2g/mL at 25°C, biology buffer
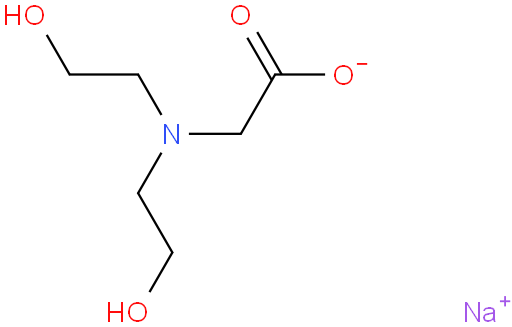
BES N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid, N,N-Bis(2-hydroxyethyl)taurine, BES, Free Acid, 2-(bis(2-hydroxyethyl)amino)ethane sulfonic acid, C6H15NO5S CAS No.10191-18-1 MW213.25 Beilstein:1781572 EC No.233-465-5 UNSPSC Code:12161700 Assay ≥99.0% (titration), form crystalline, color white, storage condition dry at room temperature, pH2.5-5.0 (25 °C, 213.3 g/L), useful pH range 6.4-7.8, pKa (25 °C)7.1, solubility water: 0.5 g/mL, clear, colorless, density 1.30 g/cm3 at 20—25 °C 1.35 g/cm3 at 20—25 °C, application(s) clinical research diagnostic assay manufacturing general analytical life science and biopharma, storage temp.room temp, General description N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid, known as BES, is a zwitterionic buffer and a valuable secondary standard in biochemical research. BES, a sulfonic acid-containing cross-linking agent, induces cross-linking between sulfonated polyimide chains in sulfonated polyimide membranes. The useful pH range for BES buffer is 6.4 to 7.8. It is one of the "Good" buffers developed in the 1960s to provide buffering capacity in the pH range of 6.15 to 8.35, making it widely applicable in biochemical studies. BES buffer is suitable for use in systems where the formation of a calcium phosphate-DNA complex is desirable (Absorbance: < 0.05 at 260 nm, 100 mM). Further, BES buffer can be employed in the study of molecular biology, biological buffers, molecular biology reagents, and zwitterionic compounds. Application BES may be employed as binding buffer in modified Eagle′s medium during the binding assay of human melanoma cells. It may be used as biobuffer to investigate the aqueous medium self-assembly of heterometallic CuII/Li 3D coordination polymers. Further, BES has found application in transfection studies, both in the preparation of reagents for transfection and in signaling assays related to Neural Melanocortin Receptors. Features and Benefits Suitable for Cell Culture media and as a Buffering solution in Biochemical and Molecular Biology Research, Effective Buffering from pH 2.5-5.0 (25 °C, 213.3 g/L) with a pKa of 7.1 (25 °C), Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications.
MOPS, 3-(N-Morpholino)propanesulfonic acid, 4-Morpholinepropanesulfonic acid, C7H15NO4S CAS NO.1132-61-2 MW209.26 Beilstein:1106776 EC No.214-478-5 UNSPSC Code:12161700, Assay ≥99.5% (titration), form crystalline powder, color white, storage condition dry at room temperature, technique(s) affinity chromatography: suitable electrophoresis: suitable, pH2.5-4 (25 °C, 209 g/L), useful pH range6.5-7.9, pKa (25 °C)7.2, solubility water: 0.5 g/mL, clear, colorless, λ 33 % in H2O, suitability suitable for HPLC, application(s) clinical research diagnostic assay manufacturing life science and biopharma, storage temp.room temp, General description MOPS (3-(N-morpholino)propanesulfonic acid) also known as Propane Sultone buffer, is a versatile "Good′s buffer" extensively employed in biological research. It shares structural similarities with MES (2-(N-morpholino)ethanesulfonic acid), featuring a morpholine ring. In terms of physiological relevance, MOPS′ pKa of 7.2 closely aligns with physiological pH, making it suitable for studies requiring a near-neutral environment. Compared to MES with a pKa of 6.1, MOPS offers a more physiologically relevant buffering capacity. MOPS serves various functions in diverse research domains. As a buffering agent, it can be used to stabilize pH in cell culture media, promoting optimal cell growth and function. Additionally, it may find application in protein purification in chromatography, stabilizes protein solutions, and acts as a reliable running buffer in various electrophoretic techniques, enabling efficient separation and analysis of biomolecules. MOPS also efficiently lyses cells for subsequent protein or nucleic acid extraction and stabilizes enzymes in solution, ensuring their activity for research purposes. It further proves valuable in fine-tuning the pH of growth media for optimal cell growth. MOPS possesses additional advantages such as minimal interaction with metal ions especially with copper (Cu), nickel (Ni), manganese (Mn), zinc (Zn), cobalt (Co) ions, making it ideal for studies involving metal ions. It exhibits excellent water solubility, facilitating its use in various buffer formulations, and minimal lipid solubility, ensuring impermeability to membranes and minimizing interference with cellular processes. In summary, MOPS is a reliable buffer widely utilized across diverse biological disciplines, distinguished by its versatility, physiological relevance, minimal metal interaction, and ease of use, with its extensive applications in cell biology, protein studies, electrophoresis, and biochemical research. Application MOPS has been used as: a cell culture additive component in lentiviral particle production, as a buffering agent in microbial growth medium and nuclei extraction buffer, as a component of the MMSE-A buffer for isolating heart mitochondria, as a component of the Synthetic Complete (SC) medium, as a buffer in fluorescence quenching of NADH. Features and Benefits Ideal for Cell Biology and Biochemical research, Can be used as a Buffer component for Electrophoresis and Protein separation, Effective Buffering from pH 6.5-7.9 (25 °C) with a pKa of 7.2 (25 °C), Highly soluble in water, Minimal metal ion binding
CHES, 2-(Cyclohexylamino)ethanesulfonic acid, C8H17NO3S CAS NO.103-47-9 MW207.29 Beilstein:2967601 EC No.203-115-6 UNSPSC Code:12161700, Assay ≥99.0% (titration), form crystalline,color white, pH3.0-5.0 (103.6 g/L), useful pH range 8.6-10.0,pKa (25 °C) 9.3, solubility water: 0.6 g/25 mL, clear, colorless, application(s) diagnostic assay manufacturing, General description CHES (2-(N-cyclohexylamino)ethanesulphonic acid) is regarded as Good′s buffers for its self-buffering and biocompatible feature. It is used in protein stabilization and does not interact with metals. Application CHES has been used: as a buffer in mannanase activity assay of antarctic fungal isolates, as a buffer for DNA digestion and labeling of 2′-deoxyadenosine 3′-monophosphate, as a component of Wilson and Horne extraction buffer for characterization of rat plasma enzymes.
CAPS, 3-(Cyclohexylamino)-1-propanesulfonic acid, C6H11NH(CH2)3SO3H CAS NO.1135-40-6 MW221.32 Beilstein:2835588 EC No.214-492-1 UNSPSC Code:12161700, Assay ≥99%, form crystalline powder, color white, pH3.0-7.0, pKa (25 °C)10.4, pKa 10.4, mp>300 °C (lit.), solubility H2O: soluble 15g + 85 mL, clear, colorless, application(s) diagnostic assay manufacturing, Application Lignin degradation by a novel thermophilic and alkaline yellow laccase from Chitinophaga sp.: This study highlights the application of CAPS buffer in stabilizing enzymes under extreme conditions for industrial lignin degradation. The use of CAPS enhances the stability and activity of laccase, pivotal for bio-refining processes (Buzzo et al., 2024). Improved Refolding of a Human IgG1 Fc (CH2-CH3) Scaffold from Its Inclusion Body in E. coli by Alkaline Solubilization.: Research demonstrates CAPS buffer′s critical role in the solubilization and refolding of recombinant proteins. This advancement is significant for therapeutic protein production, ensuring higher yield and functionality (Ishikawa et al., 2022). An optimized SDS-PAGE protocol with a new blotting system for the initial testing procedure of ESAs in doping control.: This paper utilizes CAPS buffer in the development of a new SDS-PAGE blotting system for doping tests, offering more reliable and faster detection of erythropoiesis-stimulating agents in sports (Martin et al., 2022). Insight into organophosphate chemical warfare agent simulant hydrolysis in metal-organic frameworks.: The study uses CAPS buffer to investigate the hydrolysis reactions of chemical warfare agents in metal-organic frameworks. This research is crucial for developing materials that can neutralize hazardous agents safely and efficiently (Ploskonka and DeCoste, 2019). Complexometric and argentometric titrations using thread-based analytical devices.: CAPS buffer is applied in novel thread-based analytical devices for environmental monitoring. These devices, utilizing complexometric and argentometric titrations, offer a portable, cost-effective solution for on-site water quality analysis (Jarujamrus et al., 2018).
CHAPS, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate, C32H58N2O7S CAS NO.75621-03-3 MW614.88 UNSPSC Code:12161703, Assay ≥98% (NMR), form crystalline powder, color white, mol wt micellar avg mol wt 6150, storage condition OK to freeze, desiccated (hygroscopic), aggregation number 10, color white, CMC 6 - 10 mM 6 mM(20-25°C), mp 157 °C (315 °F), transition temp cloud point >100 °C, solubility water: 1.0 M, foreign activity DNase, RNase, and Protease, none detected, shipped in ambient, storage temp.15-25°C, General description Conductivity (1.0 M, 24°C): <100 µmhos. Non-denaturing zwitterionic detergent that combines features of bile salts and N-alkyl sulfobetaines. Better at solubilizing proteins than the structurally related carboxylic acid anions, and is more effective at breaking protein-protein interactions than sodium cholate. Capable of solubilizing the opiate receptor to a state exhibiting reversible binding to opiates. Also capable of disaggregating P 450 to its monomeric form without denaturation. Has a small micellar molecular weight (6150 Daltons) and a high critical micellar concentration (6-10 mM). Can be removed from either gels or protein solutions by dialysis. Aggregation number: 4-14. Zwitterionic detergent that combines features of bile salts and N-alkyl sulfobetaines. Better at solubilizing proteins than structurally-related carboxylic acid anions and is much more effective at breaking protein-protein interactions than sodium cholate. Capable of solubilizing the opiate receptor to a state exhibiting reversible binding of opiates. Also, capable of disaggregating cytochrome P450 to its monomeric form without denaturation. Has a small micellar molecular weight and a high critical micellar concentration and can be removed from either gels or protein solutions by dialysis across a membrane. Aggregation number: 4-14. Application CHAPS is a nondenaturing zwitterionic detergent for membrane biochemistry. Useful for solubilizing membrane proteins and breaking protein-protein interactions. CHAPS′ small micellar molecular weight (6,150) and high critical micelle concentration (6-10 mM) allow it to be removed from samples by dialysis. It is also suitable for protein solubilization for isoelectric focusing and two-dimensional electrophoresis. CHAPS is commonly used for non-denaturing (without urea) IEF and has been shown to give excellent resolution of some subcellular preparations and plant proteins. Concentrations between 2-4% (w/v) are typically used in an IEF gel. Other Notes Chen, Y., et al. 1992. Biochemistry 31, 2415. Ofri, D., et al. 1992. J. Neurochem. 58, 628. Ransom, R.W., et al. 1992. Biochem. Pharmacol. 43, 1823. Simonds, W.F., et al. 1992. Proc. Natl. Acad. Sci. USA77, 4623. Yannariello-Brown, J., and Weigel, P.H. 1992. Biochemistry31, 576. Hjelmeland, L.M. 1980. Proc. Natl. Acad. Sci. USA77, 6368. Simonds, W.F., et al. 1980. Proc. Natl. Acad. Sci. USA77, 4623.

HEPES, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid), C8H18N2O4S CAS NO.7365-45-9 MW238.30 Beilstein:883043 EC No.230-907-9 UNSPSC Code:12161700, Assay ≥99.5% (titration), form crystalline powder, color white, storage condition dry at room temperature, technique(s) cell culture | mammalian: suitable, color white, pH5.0-6.5 (25 °C, 238 g/L), useful pH range6.8-8.2, pKa (25 °C)7.5, solubility water: 500 mg/mL, clear, colorless, suitability suitable for component for culture media suitable for enzyme extraction, application(s) diagnostic assay manufacturing general analytical life science and biopharma pharmaceutical, General description HEPES has been described as one of the best all-purpose buffers available for biological research. At biological pH, the molecule is zwitterionic, and is effective as a buffer at pH 6.8 to 8.2. HEPES has been used in a wide variety of applications, including tissue culture. It is commonly used to buffer cell culture media in air. HEPES finds its usage in in vitro experiments on Mg. HEPES, also known as 4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid, is a zwitterionic N-substituted aminosulfonic acid buffer. It is effective in the pH 6.8-8.2 range, with a pKa of 7.48 at 25°C. Recognized as one of the best all-purpose buffers, especially in cell biology, biochemical, and biological research, HEPES is a dipolar ionic buffer and one of the Good′s buffers that does not form significant complexes with most metal ions. This property makes it suitable as a non-coordinating buffer in solutions with metal ions. Widely utilized in cell culture, HEPES excels at maintaining physiological pH despite fluctuations in carbon dioxide concentration produced by cellular respiration, a feature not as prominent in bicarbonate buffers. It serves as an ampholytic separator for creating pH gradients in isoelectric focusing and exhibits less interference with DNA-restriction enzyme reactions compared to buffers with fewer substituted amine groups, such as Tris. Beyond these applications, HEPES may find application in various biological and biochemical studies, including immunoprecipitation, cell lysis, and live cell imaging. Its versatility makes it an indispensable tool in diverse research applications. Application HEPES has been used: As a component in Danieau medium, which is used to prevent melanin pigment formation post gastrulation of zebra-fish embryo, As a component of Hanks′ solution used in the degradation testing of Iron, As a component of HEPES medium in the transfection of cell culture with plasmids, Along with RPMI-1640, L-glutamine, FBS, Sodium pyruvate and β-mercaptoethanol for the culturing of rat insulinoma cells, As a supplement in TCM199 medium for washing fresh oocytes required for nuclear transfer studies.
MES, 2-(N-Morpholino)ethanesulfonic acid, 4-Morpholineethanesulfonic acid, CAS NO.4432-31-9 C6H13NO4S MW195.24 Beilstein:141319 EC No.224-632-3 UNSPSC Code:12161700, Assay ≥99% (titration), form powder, color white, storage condition dry at room temperature, impurities ≤1.0% water, pH2.5-4 (25 °C, 97.6 g/L), useful pH range5.5-6.7, pKa 6.1, density 1.23 g/cm3 at 20—25 °C 1.27 g/cm3 at 20—25 °C, application(s) clinical research diagnostic assay manufacturing life science and biopharma metabolomics,General description MES Buffer, recognized as one of the "Good" buffers employed in biological and biochemical applications, plays a crucial role across various research domains. This zwitterionic and morpholinic buffer proves highly valuable due to its effective buffering capacity within the pH range of 5.5 to 6.7. Notably, MES Buffer does not engage in complex formation with the majority of metals commonly employed in environmental and biological studies. Its solubility in water is excellent, and it exhibits minimal lipid solubility, rendering it impermeable to membranes. This versatile buffer may find widespread application in maintaining a stable environment in solutions dedicated to cell culture media, protein-based buffer formulations, and electrophoresis running buffers. Its significance extends to the purification processes of antibodies, peptides, proteins, blood components, and growth factors. MES Buffer stands out as an optimal choice for capillary electrochromatography, offering low ionic mobility. Notably, it serves as a safer alternative to potentially toxic agents like cacodylate and non-zwitterionic buffers, including citrate and malate. MES Buffer also proves valuable for fine-tuning the pH of growth media, stabilizing enzymatic solutions, and serving as a component of PAGE running buffer in diverse experimental settings. Application It has been utilized as a buffer bicarbonate‐containing culture medium, MES has been used as a component of the elution buffer used to elute plasma from α2-macroglobulin affinity column,It has been used as an activation buffer during antibody conjugation to microparticles, MES has been used in the functionalization of microchannels during the creation of microfluidic-integrated surface plasmon resonance (SPR) platform for pathogen detection. Features and Benefits Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications, Effective Buffering from pH 5.5-6.7 (25 °C) with a pKa of 6.1 (25 °C), Highly soluble in water, Minimal metal ion binding, Less toxic to cells than other buffers such as Tris and phosphate, Stable in a wide pH range, Low UV absorptivity and Minimal reactivity.
MES monohydrate, 2-(N-Morpholino)ethanesulfonic acid hydrate, 4-Morpholineethanesulfonic acid hydrate, C6H13NO4S·xH2O CAS NO.1266615-59-1 MW195.24 (anhydrous basis) UNSPSC Code:12161700, Assay ≥99.5% (titration), form crystalline powder, storage condition dry at room temperature, color white, useful pH range5.5-6.7, pKa 6.1, solubility water: 0.25 g/mL, clear, colorless, application(s) agriculture clinical research diagnostic assay manufacturing life science and biopharma, storage temp.room temp, General description MES Buffer, known as one of the "Good′s" buffers designed for biological applications, plays a pivotal role in various aspects of research. This zwitterionic, morpholinic buffer is particularly valuable for its buffering capabilities within a pH range of 5.5 to 6.7. MES monohydrate, finds extensive utility in maintaining a stable environment in solutions used for cell culture media, protein-based buffer formulations, and electrophoresis running buffers. Its significance extends to the purification processes of antibodies, peptides, proteins, blood components, and growth factors. Additionally, MES stands out as an excellent choice for capillary electrochromatography, offering low ionic mobility. It serves as a safer alternative to toxic agents like cacodylate and non-zwitterionic buffers such as citrate and malate. While MES excels in buffering, it′s important to note that it is not recommended for use at pH 7.4, and alternative buffers should be explored in such cases. MES Monohydrate is also used to fine-tune the pH of growth media, stabilize enzymatic solutions, and as a component of PAGE running buffer in various experimental settings. Application MES Monohydrate has been used: In the preparation of a solution used for the suspension and dilution of protoplasts to measure the density and number of protoplasts using a haemocytometer, As a component of Murashige and Skoog basal salt media, In the preparation of total ionic strength adjustment buffer (TISAB), as a constituent in mobile phase solutions employed for the analysis of high molecular weight species and charge-related variants of biopharmaceutical proteins through SEC, HIC, and IEX chromatographic techniques. Features and Benefits Suitable as a Buffer component, for Electrophoresis and Molecular Biology, Effective Buffering from pH 2.5-4.0 (25 °C, 0.5 M in H2O) with a pKa of 6.1 (25 °C), Free from DNase, NICKase, RNase, and Protease, Preparation Note A buffer using MES free acid can be prepared by titrating the free acid with sodium hydroxide to the desired pH (pKa =±1). Alternatively, volumes of equimolar MES free acid and sodium MES can be mixed to attain the desired pH. Standard mixing tables using stock solutions to prepare a buffer of a given pH have been published. Other Notes Solubility/Stability: MES is soluble in water, giving a clear colourless solution at concentrations of 0.5 M or higher. The pH of a solution should be between 2.5 and 5, depending on the concentration. Solutions are stable at 2-8C for months. Sterilization: Sterilization should be by filteration through 0.2 uM filters. Autoclaving is not recommended by any sulfonic acid buffers. If buffers must be nuclease-free, it is best to treat the water, and then add the buffer solids after autoclaving. When MES solutions are autoclaved, they turn yellow (although pH does not change measurably. The identity of the yellow breakdown product is unknown.
PIPES, 1,4-Piperazinediethanesulfonic acid, Piperazine-1,4-bis(2-ethanesulfonic acid), Piperazine-N,N′-bis(2-ethanesulfonic acid), C8H18N2O6S2 CAS NO.5625-37-6 MW302.37 Beilstein:817713 EC No.227-057-6 UNSPSC Code:12161700, Assay ≥99% (titration), form crystalline powder, color white, useful pH range6.1-7.5, pKa (25 °C)6.8, mp>300 °C (lit.), solubility 1 M NaOH: 20 + 80 mL g, clear, colorless, application(s) diagnostic assay manufacturing, General description PIPES is a member of the ethanesulfonic acid buffer series, first introduced by Good et al., developed to meet certain criteria: midrange pKa, maximum water solubility and minimum solubility in all other solvents, minimal salt effects, minimal change in pKa with temperature, chemically and enzymatically stable, minimal absorption in visible or UV spectral range and easily synthesized. Since its pKa at 37 °C is near physiological pH, PIPES has applications in cell culture work. Application Glutaraldehyde fixation of plant and animal tissue samples can cause loss of lipid, leading to apparent morphological changes. Lipid loss and artifacts were minimized when PIPES was used to buffer the glutaraldehyde fixative. Alkaline phosphatase activity is lost selectively from certain rat hepatocyte organelles when fixed for ultracytochemistry with cacodylate-buffered glutaraldehyde. When PIPES was used as buffer, retention of activity was 60% greater. Fixation of fungal zoospores for fluorescence microscopy and electron microscopy was optimal with a combination of glutaraldehyde and formaldehyde in PIPES buffer. Preparation Note Buffers can be prepared by adding a solution of base to PIPES free acid to titrate to the appropriate pH, or by mixing equimolar solutions of the monosodium salt and the disodium salt to titrate to the appropriate pH.
TES,2-[(2-Hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethanesulfonic acid, N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid, TES free acid, C6H15NO6S CAS NO.7365-44-8 MW229.25 Beilstein:1957061 EC No.230-906-3 UNSPSC Code:12161700, Assay ≥99% (titration)form crystalline powder, color white, useful pH range 6.8-8.2, pKa (25 °C)7.5,mp~225 °C (dec.),solubility H2O: 25 g plus 50 mL, clear, colorless, application(s) diagnostic assay manufacturing, Application TES has been used in TES buffer to determine anti-collagenase activity of Marula stems. It has also been used in buffers for cell culture medium.
EGTA, Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, Egtazic acid, Ethylene-bis(oxyethylenenitrilo)tetraacetic acid, Glycol ether diamine tetraacetic acid, [-CH2OCH2CH2N(CH2CO2H)2]2 CAS NO.67-42-5 MW380.35 Beilstein:1717370 EC No.200-651-2 UNSPSC Code:12352106, Assay≥97.0%, form powder, color white, reaction suitability,reagent type: chelator, loss ≤1.0% loss on drying, mp241 °C (dec.) (lit.),foreign activity, DNAse (exonuclease), NICKase (endonuclease), RNAse, and protease, none detected, Application A chelating agent useful for the determination of calcium in the presence of magnesium. Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid has been used: in Dulbecco′s phosphate-buffered saline (DPBS) to induce the weakening of cell-to-cell junctions as an extracellular calcium chelator to study the role of calcium in axonal mitochondria degeneration as a component in lysis buffer for lysing mouse mucosal cells for western blot analysis. Biochem/physiol Actions Ethylene glycol tetraacetic acid (EGTA) can chelate calcium (Ca2+) at a ratio of 1:1. It serves as an anti-coagulant when dissolved at 1 g per 100 ml of blood. EGTA can prevent the vesicular release in the nanodomain of single Ca2+ channels. This metal chelator can inhibit the activity of pseudolysin. Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid A is a chelating agent used for the determination of calcium in the presence of magnesium.

CAPSO, 3-(Cyclohexylamino)-2-hydroxy-1-propanesulfonic acid, CAPSO Free Acid, C9H19NO4S CAS NO.73463-39-5 MW237.32 Beilstein:5939234 UNSPSC Code:12161700, Assay≥99% anhydrous basis (titration), form crystalline powder, color white, pH2.5-4.0, useful pH range 8.9-10.3, pKa (25 °C)9.6, solubility water: 0.111 g/mL, clear, colorless, application(s) diagnostic assay manufacturing, General description CAPSO is a useful secondary standard buffer. Second dissociation constant pK2 value of CAPSO has been measured. Useful pH range for CAPSO is 8.9 - 10.3. Application CAPSO was used as biological buffer in the following studies: To maintain pH 9 during water leach test for the analysis of high-carbon coal fly ash-soil mixtures. Chemiluminescent in situ hybridization using soybean peroxidase-labeled peptide nucleic acid (PNA) probes. Preparation of planar bilayer lipid membranes. It may be employed as buffer for the electrophoretic analyses in non-denaturing gels, to investigate macromolecular self-association of ADP-ribosyltransferase protein in solution.
IPTG(Source Plant) PTG, Dioxane-Free, High Purity, Isopropyl-β-D-thiogalactopyranoside, is used in the stimulation of β-galactosidase in cellular systems in which dioxane would disrupt normal cell function, IPTG, Dioxane-Free, High Purity, Isopropyl-β-D-thiogalactopyranoside, C9H18O5S CAS NO.367-93-1 MW238.30 UNSPSC Code:12352201, Assay ≥98% (HPLC), 99% form solid, color white to off-white, storage condition OK to freeze desiccated (hygroscopic) protect from light, impurities ≤0.1% Dioxane (GC), solubility water: 50 mg/mL, shipped in ambient, storage temp.2-8°C, General description Inducer of β-D-galactosidase, Used in the stimulation of β-galactosidase in cellular systems in which dioxane would disrupt normal cell function, IPTG induces the expression of cloned genes linked to the lac promoter, Used in the stimulation of β-galactosidase in cellular systems in which dioxane would disrupt normal cell function, This preparation contains ≤0.1% dioxane by GC.
.jpg)
DL-1,4-Dithiothreitol, DL-Dithiothreitol, (±)-Dithiothreitol, rac-Dithiothreitol, Dithiothreitol, threo-1,4-Dimercapto-2,3-butanediol, Cleland’s reagent, DTT, HSCH2CH(OH)CH(OH)CH2SH CAS NO.3483-12-3 MW154.25 Beilstein:1719757 EC No.222-468-7 UNSPSC Code:12161504, Assay ≥98% (HPLC) ≥99% (titration) form powder, color white, reaction suitability reagent type: reductant, mp41-44 °C (lit.), solubility H2O: 50 mg/mL, cation traces heavy metals (as Pb): ≤5 ppm, suitability suitable for molecular biology, foreign activity DNase, RNase, protease, none detected, storage temp.2-8°C, General description Dithiothreitol (DTT) is an excellent reagent for maintaining SH groups in a reduced state in proteins. It initiates the thiol-disulfide exchange reaction to completely reduce the intra-or inter-molecular disulfide bonds in biomolecules. DTT specifically targets the disulfide bridge of the cross-linker N,N′-bis(acryloyl)cystamine to break apart the matrix of a polyacrylamide gel and sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE). Hence, it is highly effective in use with buffers that quantitatively reduce disulfides. DTT is less pungent and less toxic in nature than 2-mercaptoethanol. It is also 7x more concentrated than beta-mercaptoethanol. Application An excellent reagent for maintaining SH groups in reduced state; quantitatively reduces disulfides. DTT is effective in sample buffers for reducing protein disulfide bonds prior to SDS-PAGE. DTT can also be used for reducing the disulfide bridge of the cross-linker N,N′-bis(acryloyl)cystamine to break apart the matrix of a polyacrylamide gel. DTT is less pungent and is less toxic than 2-mercaptoethanol. Typically, a seven fold lower concentration of DTT (100 mM) is needed than is used for 2-mercaptoethanol (5% v/v, 700 mM). DL-Dithiothreitol is used in protein samples to reduce the disulfide bonds before loading the sample onto the gel for Western Blot or SDS-PAGE. It has been used in the procedure of in situ hybridization of mRNA. It has been used: as a component for protein extraction in western blot analysis, to prepare sample lysis buffer for quantitative mass spectroscopy, as a kinase buffer component for enzyme-linked immunosorbent assay (ELISA), Biochem/physiol Actions Dithiothreitol (DTT) is frequently utilized in biochemical preparations of thiol proteins and chemical peptide synthesis. DTT is also used in protein chemistry research on the folding of proteins and enzyme activity. Features and Benefits Suitable for molecular biology RNase, DNase, Exonuclease, and Protease-free High purity (HPLC ≥98%) No heavy metal ≤5ppm
DTE, 1,4-Dithioerythritol, erythro-1,4-Dimercapto-2,3-butanediol, erythro-2,3-Dihydroxy-1,4-butanedithiol, Cleland’s reagent, HSCH2CH(OH)CH(OH)CH2SH CAS NO.6892-68-8 MW154.25 Beilstein:1719756 EC No.229-998-8 UNSPSC Code:12161504, grade Molecular Biology, product line BioReagent, Assay ≥99.0%(titration with iodine), 1H NMR spectrum conforms to structure, form powder, color white, reaction suitability, reagent type: reductant, pH 4.5-6.5 (25 °C, 15.4 g/L), mp 82-84 °C (lit.), solubility water: 50 mg/mL, foreign activity DNase, Exonuclease detection, RNase, and protease, none detected, storage temp.2-8°C, Application Reagent for maintaining −SH groups in the reduced state; quantitatively reduces disulfides.

X-GAL, 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside, BCIG, C14H15BrClNO6 CAS NO.7240-90-6 MW408.63 UNSPSC Code:12352202, Assay 99%, form powder, storage condition OK to freeze, color white, shipped in ambient, General description X-Gal is a chromogenic substrate used in conjunction with IPTG for blue/white colony selection. It is a soluble colorless compound comprising galactose attached to a substituted indole. In the presence of X-Gal the lac+ bacteria after α-complementation form blue colonies and bacteria carrying recombinant plasmid form white colonies.
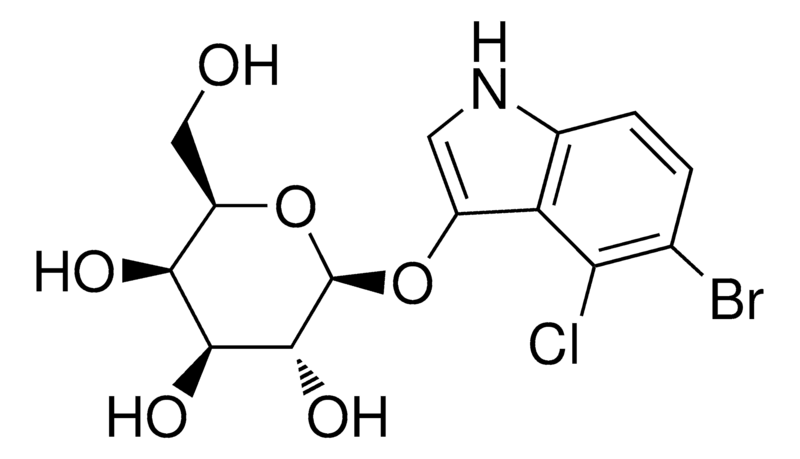
X-Gluc, X-GlcA, reagent for selection of recombinant bacterial clones, 5-Bromo-4-chloro-3-indolyl β-D-glucuronide cyclohexylammonium salt, X-GlcA, X-glucuronide CHA salt, C14H13BrClNO7·C6H13N CAS NO.114162-64-0 MW521.79 UNSPSC Code:12352200, grade Molecular Biology, sterility non-sterile, Assay ≥98% (TLC), form powder, color White to Almost white powder to crystal, technique(s) nucleic acid detection: suitable, solubility DMF: soluble, suitability suitable for β-galactosidase test, storage temp. −20°C, General description X-GlcA is a chromogenic substrate for β-galactosidase, used to determine the presence or absence of a cloned DNA insert in bacteria growing on agar plates. X-GlcA is designed to replace X-Gal in blue-white selection of recombinant bacterial colonies with the lac+ phenotype. Application Chromogenic substrate for β-glucuronidase (GUS) gene detection. Suitable for use in selection of recombinant bacterial colonies with the lac+ phenotype. X-GlcA has been used in histochemical staining of root sections for light microscopic observation. Principle When X-GlcA is hydrolyzed by β-galactosidase, the resulting product will form a blue precipitate. Lac+ colonies grown in the presence of X-GlcA turn an intense blue color, allowing for easy differentiation between lac+ and lac- colonies.
ONPG 2-Nitrophenyl β-D-galactopyranoside, ONPG , o-Nitrophenyl β-D-galactopyranoside, ONPG, Notation), C12H15NO8 CAS Number:369-07-3 MW301.25 Beilstein:92207 EC No.206-716-1 UNSPSC Code:12352200, grade Molecular Biology, Assay ≥99% (enzymatic), form powder, color white to off-white to light yellow powder, storage temp.−20°C, General description ONPG (2-Nitrophenyl β-D-galactopyranoside) is a colorimetric substrate for β-galactosidase. Application 2-Nitrophenyl β-D-galactopyranoside is an enzyme substrate used to detect lacZ activity and hence the presence of β-galactosidase. Biochem/physiol Actions β-galactosidase, breaks down lactose into galactose and glucose. β-Galactosidase is not lactose specific and can act on simple galactosides. 2-Nitrophenyl β-D-galactopyranoside hydrolysis results in the release of galactose and a yellow chromogenic compound. The test substrate does not depend on an induced or constitutive permease enzyme to enter the cell, therefore reactions are rapid and occur within a 24-hour period. Principle β-galactosidase, breaks down lactose into galactose and glucose. β-Galactosidase is not lactose specific and can act on simple galactosides. 2-Nitrophenyl β-D-galactopyranoside hydrolysis results in the release of galactose and a yellow chromogenic compound. The test substrate does not depend on an induced or constitutive permease enzyme to enter the cell, therefore reactions are rapid and occur within a 24-hour period. Reconstitution A stock solution can be prepared in molecular biology grade water at a concentration of 3 mg/ml. Alternatively, a stock solution of approximately 20.5 mg/ml is prepared in 100 mM sodium phosphate buffer (pH 7.3). Gentle warming may be required to completely dissolve the product. Substrates Chromogenic substrate for β-galactosidase
2-Nitrophenyl β-D-glucopyranoside, 2-Nitrophenyl-beta-D-glucopyranoside, C12H15NO8 CAS NO.2816-24-2 MW301.25, Assay ≥98%, form powder, color light yellow, mp140-141 °C(lit.) bp572.4 °C(lit.), solubility soluble in water, storage sealed in cool and dry environment. Applications 2-Nitrophenyl-beta-D-glucopyranoside is used as a reaction substrate for glucosidase
PNPG, 4-Nitrophenyl-beta-D-galactopyranoside(pNPG), C12H15NO8 CAS NO.3150-24-1 MW301.25 assay 99%, form crystalline powder, color White to light yellow, mp 178-182 °C(lit.) bp 442.39°C(lit.) solubility Soluble in DMSO, Methanol, Water, storage conditions Inert atmosphere, Stored in freezer, under -20°C, Applications PNPG is the Substrate for beta-galactosidase.
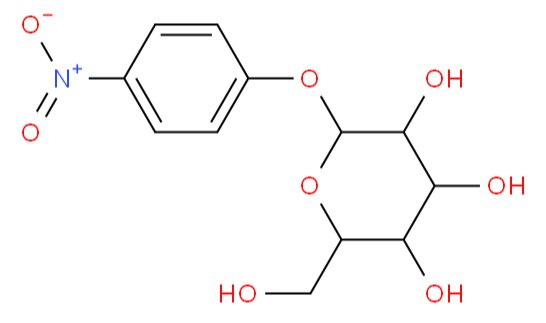
D-Glactose, D-(+)-Galactose, C6H12O6 CAS NO.59-23-4 MW180.16 Beilstein:1724619 EC No.200-416-4 UNSPSC Code:12352201powder, Assay ≥99% (HPLC), form powder, color white, technique(s): cell culture | insect: suitable, cell culture | mammalian, impurities ≤0.1% glucose, useful pH range, 5.0-7 (25 °C, 180 g/L), mp 168-170 °C (lit.), solubility H2O: soluble 100 mg/mL, clear, colorless, cation traces Mn: ≤50 ppb, storage temp. room temp, General description Galactose is a constituent of lactose. It is one of the main nutrients for newborn infants and young children.[1] It is produced in the body for the formation of lactose and is also a component of glycolipids (cerebrosides) and glycoproteins. Application D-(+)-Galactose has been used in cell line and culture medium to permits the cell growth arrest without altering the specific production rate of rh-tPA (qtPA), D-(+)-Galactose has been used in cell line and culture medium to permits the cell growth arrest without altering the specific production rate of rh-tPA (qtPA).
β-D-Galactose Pentaacetate, 1,2,3,4,6-Penta-O-acetyl-β-D-galactopyranose, Penta-O-acetyl-β-D-galactopyranose, C16H22O11 CAS NO.4163-60-4 MW390.34 Beilstein:98853 EC No.224-008-0 UNSPSC Code:12352201, Assay 99%(GC), form powder, color white, optical activity [α]20/D +8.6°, c = 1.5% in chloroform [α]24/D +23°, c = 1 in chloroform, mp143-144 °C (lit.), solubility methanol: 50 mg/mL, clear, Applications Selective 1-O-deacetylation, Selective β-1-C-allenylation, Stereoselective β-thioglycosylation. Storage Store at -20℃, protected from light, in a well-ventilated dry place, sealed.
alpha-D-Galactopyranose 1,2,3,4,6-pentaacetate,α-D-Galactose pentaacetate,C16H22O11 CAS NO.4163-59-1 MW390.34 assay 95%, appearance: Powder White to Off-white, mp95-96 °C
α-D-Glucopyranoside, methyl, Methyl α-D-glucoside,AMG, 99%(GC) C7H14O6 CAS No.97-30-3 MW194.18 EC No.202-571-3 Beilstein No.81568 UNSPSC Code:12352201, Assay 99%(GC), form solid, appearance crystalline powder, mp 168 °C(lit.), bp:200 °C (0.2 mmHg), optical activity: [α]20/D 156 to 160 °, c = 10% (w/v) in water, technique(s) gas chromatography (GC): suitable, color white, solubility water: 50 mg/mL, clear colorless, storage temp.room temp
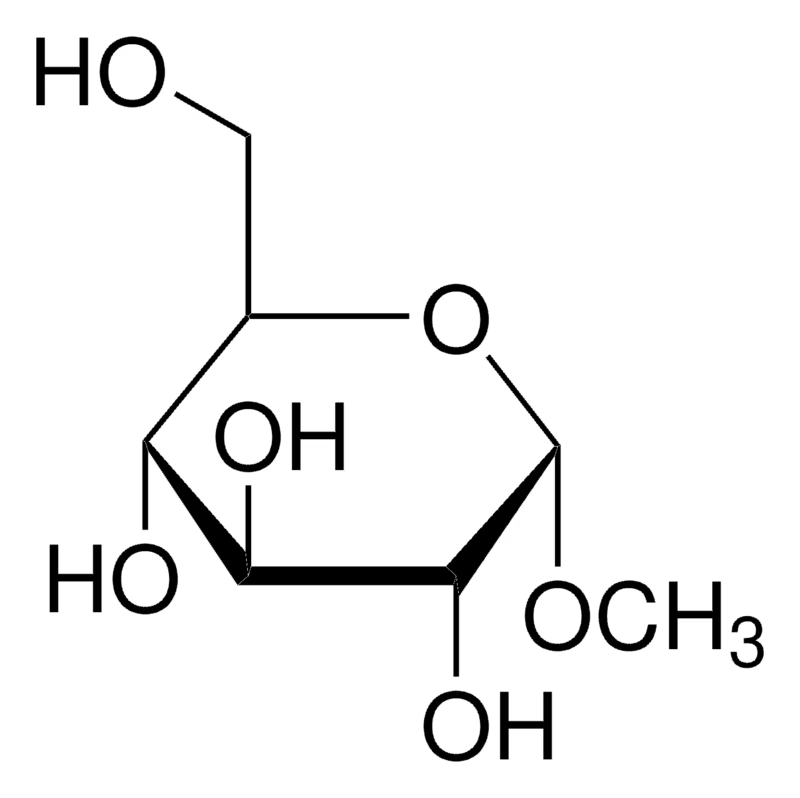
Lactobionic acid, C12H22O12 CAS NO.96-82-2 MW358.3, form Powder, color white, 1 H NMR Spectrum: Consistent with structure, Assay (NMR): ≥98.0%, mp113-118 °C(lit.), bp410.75°C (rough estimate), Lactobionic Acid is used in solutions to preserve organs involved in surgical transplants. Also a potential inhibitor of Trypanosoma cruzi as a lactose derivative. [1]. Sumimoto, R. et al.: Transplant., 53, 1206 (1992); [2]. Mitchell, S. et al.: Cryobiol., 33, 413 (1996); [3]. Agusti, R. et al.: 14, 659 (2004);
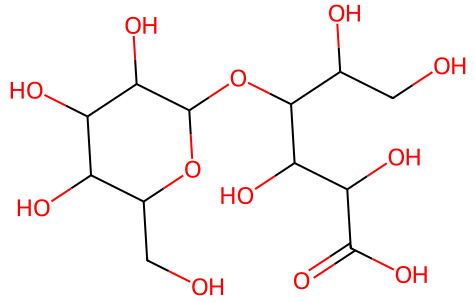
Methyl-α-D-Mannopyranoside, Methyl α-D-mannopyranoside,α-Methyl-D-mannoside is a substrate for α 1,2-Mannosyltransferase C7H14O6 CAS NO.617-04-9 MW194.18 UNSPSC Code:12352201,Assay:≥99% (TLC) form:solid, storage condition:do not freeze, color:white to off-white, solubility water: soluble,cation traces heavy metals: ≤10 ppm, shipped in ambient, storage temp.10-30°C

4-Nitrophenyl-α-D-Glucopyranoside , α-glucosidase Chromogenic substrate, p-Nitrophenyl-α-D-glucopyranoside, C12H15NO8 CAS NO.3767-28-0 MW301.25 UNSPSC Code:12352200,Assay ≥99% (HPLC), form solid powder, storage condition OK to freeze, impurities ≤0.3% p-nitrophenol, color white to off-white, solubility water: 10 mg/mL, mp204-218°C(lit.), bp442.39°C(lit.), shipped in ambient, storage temp.−20°C
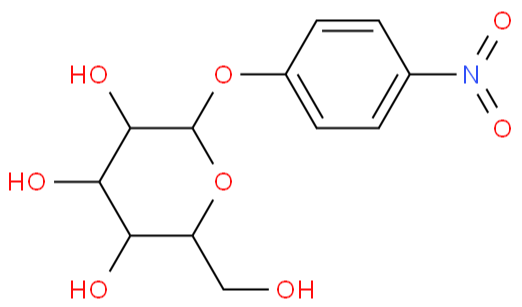
4-Nitrophenyl-α-D-Galactopyranoside, 4-NITROPHENYL-ALPHA-D-GALACTOPYRANOSIDE, it can bind to the lactose protein carrier of E. coli and can be added to the α-D-lactose medium, C12H15NO8 CAS NO.7493-95-0 MW301.25, Assay 99% form powder, solubility methanol: 100 mg/mL, clear to very slightly hazy, mp166-169 °C(lit.), bp582.2±50.0 °C(lit.), storage conditions −20°C
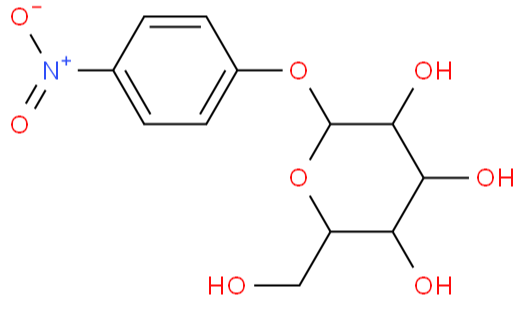
Gellangum, Gellan Gum, Agar substitute gelling agentBioReagent, suitable for plant cell culture, CAS NO.71010-52-1 EC No.275-117-5 UNSPSC Code:10171502, form powder, appearance white, technique(s) cell culture | plant: suitable, application(s) agriculture

Cholesterol, 3β-Hydroxy-5-cholestene, 5-Cholesten-3β-ol, C27H46O CAS NO.57-88-5 MW386.65 Beilstein:1915888 EC No.200-353-2 UNSPSC Code:12352111, biological source sheep wool, Assay ≥99%, form powder, bp360 °C (lit.), mp147-149 °C (lit.), solubility water: soluble 0.00003 g/L at 20 °C, density 1.067 g/mL at 25 °C (lit.), shipped in ambient, storage temp.−20°C
(+)-Abscisic acid, ABA, (+)-Abscisic Acid, (+)-cis,trans-Abscisic Acid, S-ABA, C15H20O4 CAS NO.21293-29-8 MW264.32 UNSPSC Code:12352106, Assay ≥98% (HPLC), form powder, storage condition OK to freeze protect from light, color off-white, solubility DMSO: 100 mg/mL, storage temp.−20°C
-Abscisic acid.png)
Casein, from bovine milk, suitable for substrate for protein kinase (after dephosphorylation), purified powder, CAS NO.9000-71-9 EC No.232-555-1 UNSPSC Code:12352202, assay 92%, biological source bovine milk, form purified powder yellow to tan purified powder, technique(s) activity assay: suitable electrophoresis: suitable immunocytochemistry: suitable, mp280 °C (dec.) (lit.), suitability suitable for substrate for protein kinase (after dephosphorylation)
Sodium caseinate, CAS NO.9005-46-3 UNSPSC Code:12352202, biological source bovine milk, form powder, technique(s) electrophoresis: suitable immunocytochemistry: suitable ligand binding assay: suitable, color white to yellow, solubility H2O: soluble 50 mg/mL, Grade III

β-Glycerol phosphate disodium salt,β-Glycerol phosphate disodium salt pentahydrate,β-GP, β-glycerolphosphate, Disodium β-glycerol phosphate pentahydrate, glycerol-2-phosphate, C3H7Na2O6P·5H2O CAS NO.13408-09-8 MW306.11 Beilstein:3744328 EC No.212-464-3 UNSPSC Code:41141710, Assay ≥98.0% (NT), form solid, impurities ≤2% L-α-glycerol phosphate, loss 26-32% loss on drying, solubility H2O: 0.1 g/mL, clear, colorless, storage temp.2-8°C, mp102°C(lit.), bp300°C(lit.)
Calcium fructoborate, Monocalcium mono((3aR,3a'R,5R,5'R,6S,6aS,6'S,6a'S)-6,6'-dihydroxy-3a,3a',5,5'-tetrakis(hydroxymethyl)octahydro-2,2'-spirobi[furo[2,3-d][1,3,2]dioxaborol]-2-uide), C24H40B2CaO24 CAS NO.250141-42-5 MW774.26, form Solid, color White to off-white, Assay (NMR) 98%, pH 6.0-8.0, Water: ≤5.0%, Ca: ≤5.0%, Oxides: ≤0.05%, Pb: non-detected, aldehyde: ≤1ppm, heavy metals: ≤1ppm

Calcium levulinate, Calcium levulinate dihydrate, γ-Ketovaleric acid calcium salt, 4-Oxopentanoic acid calcium salt, Levulinic acid calcium salt dihydrate, CH3COCH2CH2COO·1/2Ca·H2O CAS NO.5743-49-7 MW153.16 Beilstein:3764203 UNSPSC Code:41116107, Assay 98%,grade pharmaceutical primary standard, API family calcium levulinate, mol wt monograph mol wt. 306.32 (C10H14CaO6.2H2O), manufacturer/tradename USP, application(s) pharmaceutical (small molecule), format neat, appearance white, form powder, mp123 °C(lit.)
Agarose, BioReagent, Molecular Biology, low EEO, 3,6-Anhydro-α-L-galacto-β-D-galactan, Agarose LE, CAS NO.9012-36-6 EC No.232-731-8 UNSPSC Code:41105317, biological source algae (marine), grade Molecular Biology, product line BioReagent, form powder, color white to light yellow, technique(s) electrophoresis: suitable, impurities ≤10% moisture content, EEO 0.09-0.13, transition temp gel point 36 °C ±1.5 °C (1.5% gel), gel strength ≥1200 g/cm2 (1% gel), solubility soluble in water, anion traces sulfate (SO42-): ≤0.15%, suitability suitable for electrophoresis suitable for molecular biology, foreign activity DNase, RNase, none detected, mp90°C(lit)
D(+)-Trehalose, D-(+)-Trehalose dihydrate, from Saccharomyces cerevisiae, Trehalose dihydrate, α,α-Trehalose, α-D-Glucopyranosyl-α-D-glucopyranoside ≥99% (HPLC), C12H22O11·2H2O CAS NO.6138-23-4 MW378.33 Beilstein:5322018 EC No.202-739-6 UNSPSC Code:12352201, biological source Saccharomyces cerevisiae, Assay ≥99% (HPLC), form powder, optical activity [α]20/D 174 to 182 °, c = 7% (w/v) in water, technique(s) HPLC: suitable PCR: suitable cell based assay: suitable cryopreservation: suitable, impurities ≤5.0% EtOH <20 ppm Trace metals, color white, mp 97-99°C, solubility water: 50 mg/mL, clear, colorless, application(s) advanced drug delivery agriculture cell analysis life science and biopharma sample preparation, storage temp.room temp
-Trehalose.png)
D(-)-Fructose, D-Levulose, Fruit sugar, C6H12O6 CAS NO.57-48-7 MW180.16 Beilstein:1239004 EC No.200-333-3 UNSPSC Code: 12352201, biological source corn, Assay ≥99% (HPLC), form powder, technique(s) HPLC: suitable, impurities ≤0.05% Glucose (enzymatic), color colorless, useful pH range 5.0-7 (25 °C, 18 g/L), mp119-122 °C (dec.) (lit.), bp232.96°C(lit.) solubility water: 790 g/L at 20 °C, storage temp.room temp
-Fructose.png)
Gelatine from cold water fish skin, powder, suitable for cell culture and generation of scaffolds. Gelatine, Teleostean gelatin, CAS NO.9000-70-8 EC No.232-554-6 UNSPSC Code:12352202, biological source fish skin, product line BioReagent, form solid, color yellow to off-white, mol wt 60 kDa, technique(s) cell culture | mammalian: suitable, surface coverage 100—200 μg/cm2, impurities ≤2% Residue on ignition (Ash) ≤20 ppm Heavy metals, solubility acetic acid: soluble glycerol: soluble water: soluble, shipped in ambient, storage temp.room temp

D-Mannose, D-(+)-Mannose, D-Mannopyranose, (1)BioReagent, suitable for cell culture, (2)99%(GC), C6H12O6 CAS NO.3458-28-4 MW180.16 Beilstein:1564373 EC No.222-392-4 UNSPSC Code:12352201, biological source plant (Spruce, Birch or Beech Wood, Glucose), product line BioReagent, Assay ≥99%, form powder, color White to off-white, 1 H NMR Spectrum: Consistent with structure, technique(s) cell culture | mammalian: suitable, mp133-140 °C (lit.), bp410.8°C at 760 mmHg, solubility H2O: 50 mg/mL, Storage: 2-8°C, stored under nitrogen, application(s) agriculture, D-Mannose is a carbohydrate that is important in the glycosylation of molecules in a variety of cellular processes. It is involved in N and O glycosylation of bovine why protein products, used in infant formulas. It is also responsible for the O-glycosylation of the T helper cell-derived cytokine interlukin-17A, an important cell-signaling molecule. [1]. van Leeuwen, S. et al.: J. Agri. Food Chem., 60, 12553 (2012); Geoghegan, K. et al.: Prot. Express. Purif., 87, 27 (2013);
L-Glutathione oxidized, L(-)-Glutathione, GSSG, Glutathiol, C20H32N6O12S2 CAS NO.27025-41-8 MW612.63 Beilstein:1718700 EC No.248-170-7 UNSPSC Code:12161501, Assay ≥98% (HPLC), form powder, color white, solubility H2O: soluble, storage temp.2-8°C
L-Glutathione(Reduced Form), Glutathione, Reduced, γ-L-Glutamyl-L-cysteinyl-glycine, GSH, H2NCH(CO2H)CH2CH2CONHCH(CH2SH)CONHCH2CO2H CAS NO.70-18-8 MW307.32 Beilstein:1729812 EC No.200-725-4 UNSPSC Code:12352209, Assay ≥98.0%, form powder, color white, technique(s) cell culture | mammalian: suitable, mp192-195 °C (dec.) (lit.), solubility H2O: 50 mg/mL, storage temp.2-8°C
.png)
D(+)-Raffinose pentahydrate, D-(+)-Raffinose pentahydrate O-α-D-Galactopyranosyl-(1→6)-α-D-glucopyranosyl β-D-fructofuranoside, Melitose, Melitriose, C18H32O16·5H2O CAS NO.17629-30-0 MW594.51 Beilstein:99541 EC No. 208-146-9 UNSPSC Code:12352201, biological sourceplant (Gossypium hirsutum L./Cotton), Assay ≥99.0% (HPLC), form powder, technique(s) HPLC: suitable, color white, solubility water: 50 mg/mL, clear, colorless, storage temp.room temp
-Raffinose pentahydrate.png)
D-Arabinose, D-(-)-Ababinose, D(–)Arabinose, C5H10O5 CAS NO.10323-20-3 MW150.13 UNSPSC Code:12352201, Assay ≥99% (HPLC), form solid, storage condition OK to freeze, color white, solubility water: 100 mg/mL, mp156-160 °C(lit.), solubility: DMSO (Slightly), Water (Sparingly), shipped in ambient, storage temp.15-25°C, D-Arabinose is a reducing sugar. It is a pentose analog of D-ribose and a component of the arabinogalact of the mycobacterial cell wall. It is also a substrate for the synthesis of D-erythroascorbic acid in yeast. [1]. A F Hernández-Pérez, et al. Biochemical conversion of sugarcane straw hemicellulosic hydrolyzate supplemented with co-substrates for xylitol production. Bioresour Technol. 2016 Jan:200:1085-8. [2]. Fan Li, et al. Structure and bioactivity of a polysaccharide extracted from protocorm-like bodies of Dendrobium huoshanense. Int J Biol Macromol. 2015 Jan:72:664-72. [3]. Fei Huang, et al. Antioxidant and antiproliferative activities of polysaccharide fractions from litchi pulp. Food Funct. 2015 Aug;6(8):2598-606. [4]. Gabriel B Akanni, et al. Protein enrichment of an Opuntia ficus-indica cladode hydrolysate by cultivation of Candida utilis and Kluyveromyces marxianus. J Sci Food Agric. 2015 Mar 30;95(5):1094-102. [5]. Jun Zhou, et al. Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice. J Ethnopharmacol. 2015 Apr 22:164:229-38. [6]. Chen, X., et al.: Int. J. Biol. Macromol., 104, 1294 (2017); Konuchova, M., et al.: Chemicke Listy, 110, 491 (2016); Pelckmans, M., et al.: ACS Catalysis, 8, 4201 (2018); Haghparast, S., et al.: J. Agric. Sci. Techn., 15, 87 (2013)
L-Arabinose, L-(+)-Arabinose,Aldehydo-L-arabinose,C5H10O5 CAS NO.5328-37-0 MW150.13 Beilstein:1723085 EC No.226-214-6 UNSPSC Code:12352201, Assay ≥99% (GC), form powder, optical activity [α]/D +103 to +105°(lit.), color white, mp160-163 °C (lit.), solubility water: 100 mg/mL, clear, colorless

Salecan, Beta-Glucan, CAS NO.1439905-58-4, Assay 90%, color white or off-white form powder

Chitosan(98%), Deacetylated chitin, Poly(D-glucosamine), (C6H11NO4)n CAS NO.9012-76-4 MW n(161.16) UNSPSC Code:12352201, high molecular weight, form (Coarse ground flakes and powder), color white, mol wt 310000-375000 Da, viscosity 800-2000 cP, 1 wt. % in 1% acetic acid(25 °C, Brookfield)(lit.), solubility H2O: insoluble organic solvents: insoluble, mp102.5 °C(lit.), biotech grade

Glycogen, (C6H10O5)n CAS NO.9005-79-2 MW(666.578)n, EC No.232-683-8, UNSPSC Code:12352201, form powder, color Off-white, mp270-280 °C (lit.) solubility H2O: 20 mg/mL, clear to hazy, faintly yellow, Application This preparation is used as a carrier for the precipitation of nucleic acids (DNA or RNA). As an inert material it may replace tRNAs or sonicated DNAs. 20 μg glycogen (1 μl solution) allow to precipitate pg-amounts of DNA or RNA from a volume of 1 ml. In a typical experiment 5 pg [3H]-labeled calf thymus DNA were dissolved in 500 μl 10 mM Tris-HCl, pH 8.0; 1 mM EDTA; 0.4 M LiCl. 1μl glycogen solution (20 μg glycogen) as carrier was added and then precipitated with 1.2 ml ethanol at -15 to -25 °C and stored for 3 hours at -15 to -25 °C. After centrifugation (10 minutes at 12 000 × g) the total radioactivity was found in the precipitate. Without addition of glycogen no precipitation of DNA occured. Features and Benefits Glycogen, in special quality for molecular biology, is an inert carrier in nucleic acid preparations. Contents Aqueous solution, 20 mg/ml. Analysis Note Tested for absence of endonucleases, nicking activity, exonucleases, RNases, nucleic acids, and proteases according to the current Quality Control procedures. Other Notes For life science research only. Not for use in diagnostic procedures.

D-(+)-Cellobiose, β-D-Glc-(1→4)-D-Glc, 4-O-β-D-Glucopyranosyl-D-glucose, C12H22O11 CAS No.528-50-7 MW342.30 EC No.:208-436-5 Beilstein No.:93795 UNSPSC Code:12352201, Assay ≥98% (HPLC), form powder, color White to Off White beige, optical activity [α]/D 34±1 deg, c = 10% (w/v) in water, technique(s) HPLC: suitable, mp239 °C (dec.) (lit.), solubility water: 50 mg/mL, clear, colorless, storage temp.room temp, General description Cellobiose, a disaccharide, is made up of two d-glucose molecules linked by a β-1,4-glycosidic bond. This reducing sugar can mutarotate and is produced by hydrolysis of cellulose. Application D-(+)-Cellobiose has been used: as a component in test sugar solution for cellobiose-mannitol permeability test, in the preparation of lyophilization solutions to study its ability to protect lyophilized β-galactosidase from enzymatic activity loss and secondary structure changes during storage, as a fermentation/growth substrate to grow Clostridium thermocellum to study its impacts on the qualitative and quantitative changes in cellulosome composition.
-Cellobiose.png)
Xylene Cyanole FF, Xylene Cyanol FF, Acid blue 147, Cyanol FF, XC C25H27N2NaO6S2 CAS NO.2650-17-1 Molecular Weight:538.61 Colour Index Number:42135 EC No.220-167-5 UNSPSC Code:12171500, grade Molecular Biology, Dye Content 75%, product line BioReagent, form powder,color Light Green to Very Dark Green and Purple to Very Dark Purple and Black, technique(s) nucleic acid detection: suitable,mp295 °C(lit.) solubility H2O: complete 1 mg/mL, clear, blue, suitability suitable for nucleic acid staining,storage temp.room temp, General description Xylene cyanol is often used as a tracking dye during agarose or polyacrylamide gel electrophoresis. It has a slight negative charge and will migrate the same direction as DNA, allowing the user to monitor the progress of molecules moving through the gel. The rate of migration varies with gel composition. Application Xylene Cyanol FF is used as: histological stain and as tracker dye in DNA sequencing and electrophoresis, gel-loading dye formamide RNA loading dye, tracking dye in native polyacrylamide gel electrophoresis (PAGE) loading buffer, Biochem/physiol Actions Xylene Cyanol FF acts as nucleic acid stain. Reconstitution A 6x Gel Loading Buffer can be made using standard recipes, including 0.05% xylene cyanol.

Hematoxylin, Natural Black 1, C16H14O6·xH2O CAS NO.517-28-2 MW302.28 (anhydrous basis) Colour Index Number:75290 Beilstein:91400 EC No.208-237-3 UNSPSC Code:12352204 mp200 °C (dec.)(lit.) bp363.32°C (rough estimate) Assay 99%, form powder, color Faint Yellow to Yellow and Faint Orange to Orange and Faint Beige to Beige and Faint Brown to Brown, technique(s) titration: suitable, solubility 95% ethanol: 1 mg/mL, Application Hematoxylin has been used for staining gastric cancer tissues, myocardial tissue, heart sections and ovarian nuclei. Biochem/physiol Actions Hematoxylin is an organic stain which chelates with metal ions and binds to DNA and RNA. It is used for staining nucleus of the cells. Hematoxylin along with eosin stain is used in the diagnosis of invasion stage in tumors.

Phenol Red, (C6H4OH)2C7H4SO3 CAS Number:143-74-8 MW354.38 UNSPSC Code:12171502 EC Index Number:205-609-7, Assay 98.0-102.0% (bromatometric, calculated on dried substance), form solid powder, color dark red to dark brown, NMR Conforms to Structure, MS Conforms to Structure, UV Absorbance 193 nm, 264 nm, 432 nm, loss ≤1.0% loss on drying, 105°C, mp>300 °C, bulk density 200‑300 kg/m3, Solubility: DMSO(Slightly), Methanol (Very Slightly), storage temp.no temp limit, Application Antitussive, Expectorant and Antipyretic Effect of the Ethanolic Extract of the Leaves of Momordica charantia L.: This study investigates the application of phenol red in assessing the bioactivities of plant extracts, demonstrating the versatility of phenol red in chemical assays (Ribeiro et al., 2024). Chemical Analysis by LC-MS of Cannabis sativa Root Samples from Northeast Brazil and Evaluation of Antitussive and Expectorant Activities.: Phenol red was used as part of the LC-MS methodology to analyze plant roots, highlighting its role in modern analytical techniques (Menezes et al., 2022). Quantifying Sample Collection and Processing Impacts on Fiber-Based Tear Fluid Chemical Analysis.: Phenol red is utilized to quantify changes in tear fluid composition, underscoring its importance in clinical chemical analysis (Barmada & Shippy, 2020). The In Vitro Activity of Essential Oils against Helicobacter Pylori Growth and Urease Activity.: This research uses phenol red in enzyme inhibition studies, contributing to the understanding of antimicrobial properties of essential oils (Korona-Glowniak et al., 2020). Analysis Note Assay (bromatometric, calculated on dried substance): 98.0 - 102.0 %, Identity (UV/VIS-Spectrum): passes test, Identity (Color transition): passes test, Identity (Bromination): passes test, Insoluble substances: ≤ 0.5, Sulfated ash: ≤ 0.2, Loss on Drying (105°C): ≤ 1.0, [1]. Anne Theron, et al. Novel in silico-designed estradiol analogues are cytotoxic to a multidrug-resistant cell line at nanomolar concentrations. Cancer Chemother Pharmacol. 2015 Feb;75(2):431-7. [2]. Boya Feng, et al. Methionine oxidation accelerates the aggregation and enhances the neurotoxicity of the D178N variant of the human prion protein. Biochim Biophys Acta. 2014 Dec;1842(12 Pt A):2345-56. [3]. Filippa Pettersson, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res. 2015 Mar 15;75(6):1102-12. [4]. John A Marwick, et al. Oxidants induce a corticosteroid-insensitive phosphorylation of histone 3 at serine 10 in monocytes. PLoS One. 2015 Apr 23;10(4):e0124961. [5]. Kazuhisa Hashiba, et al. Galectin-3 contributes to luteolysis by binding to Beta 1 integrin in the bovine corpus luteum. Biol Reprod. 2014 Jul;91(1):2.

Phenol Red Sodium Salt, Phenolsulfonephthalein sodium salt, C19H13NaO5S CAS Number:34487-61-1 MW376.36 Beilstein:6260026 EC No.252-057-8 UNSPSC Code:12171500, Assay 85%(HPLC), form powder or crystals, technique(s) titration: suitable, color red to brown to very dark red, visual transition interval 6.8-8.2, yellow to red, mp285 °C (dec.) (lit.), solubility water: 1 mg/mL, opaque, dark red, εmax 31.62 at 423 nm in 0.1 M phosphate buffer, application(s) diagnostic assay manufacturing hematology, histology, General description Phenol Red sodium salt is widely used as a pH indicator. At a pH of 6.4 and below, phenol red solution will have a yellow color. At a pH of 8.2 and above, it will have a red color. It is commonly used in culture media to detect neutral to acidic pH changes. Application Phenol Red sodium salt has been used as an indicator during single-guide RNA microinjection into one-cell stage zebrafish embryos. Suitable for use as a pH indicator.
Bromophenol Blue, 3′,3′′,5′,5′′-Tetrabromophenolsulfonephthalein, Bromophenol Blue (BPB), Bromphenol Blue Sultone Form, 3′,3″,5′,5″-Tetrabromophenolsulfonphthalein, C19H10Br4O5S CAS NO.115-39-9 MW669.96 UNSPSC Code:12171502 EC Index Number:204-086-2, form solid,color light pink to purple, solubility soluble in acetic acid, loss ≤1% loss on drying, 110°C, visual transition interval 3.0-4.6, greenish yellow to blue-violet, mp270-273 °C (decomposition),bulk density 730 kg/m3, storage temp.2-30°C, Application Non-targeted analysis and toxicity prediction for evaluation of photocatalytic membrane distillation removing organic contaminants from hypersaline oil and gas field-produced water.: The study uses 2-Butanone in experimental setups to assess the removal of hazardous organic compounds in water treatment processes, highlighting its role in environmental safety and sustainability (Delanka-Pedige et al., 2024). Lipid and volatile profiles of Finnish oat batches of pure cultivars: Effect of storage on the volatile formation.: This research utilizes 2-Butanone for the extraction and analysis of volatile compounds in oats, which helps in understanding the effects of storage conditions on food quality and safety (Puganen et al., 2024). [1]. Brett Deml, et al. Mutations in MAB21L2 result in ocular Coloboma, microcornea and cataracts. PLoS Genet. 2015 Feb 26;11(2):e1005002. [2]. Charmaine J Green, et al. Insulin-like growth factor 1 increases apical fibronectin in blastocysts to increase blastocyst attachment to endometrial epithelial cells in vitro. Hum Reprod. 2015 Feb;30(2):284-98. [3]. Filippa Pettersson, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res. 2015 Mar 15;75(6):1102-12. [4]. Keijiro Hara, et al. Blocking of the interaction between Wnt proteins and their co-receptors contributes to the anti-tumor effects of adenovirus-mediated DKK3 in glioblastoma. Cancer Lett. 2015 Jan 28;356(2 Pt B):496-505. [5]. Maja Segvi Klari, et al. Disturbed Hsp70 and Hsp27 expression and thiol redox status in porcine kidney PK15 cells provoked by individual and combined ochratoxin A and citrinin treatments. Food Chem Toxicol. 2014 Sep:71:97-105. [6] Inorganica Chimica Acta., vol 368, #1 p. 223-230. [7] Chemosphere, vol. 36 #6 p. 1445-1452. [8] Canadian Journal of Chemistry, vol. 69, #6 p. 937-944.

Cresol Red, o-Cresolsulfonphthalein, C21H18O5S CAS NO.1733-12-6 MW382.43 Beilstein:343399 EC No.217-064-2 UNSPSC Code:12171500, grade indicator grade, form powder or crystals, composition Dye content, 95%, technique(s) titration: suitable, color red-brown, pH 7.2-8.8, yellow to red, useful pH range 1.8-2.0, orange to yellow(acid) 7.0-8.8, yellow to violet(alkaline), mp290 °C, bp479.59 °C(ca.), density 0.998 g/cm3, λmax 367 nm (2nd)570 nm, solubility soluble in water, application(s) diagnostic assay manufacturing hematology histology, storage temp.room temp, Cresol Red, also known as o-cresol red, belongs to the phthalein and sulphonphthalein group of dyes. Cresol red changes color from orange to yellow at pH between 0.2-1.8 and yellow to violet at pH between 7.0-8.8. In alkaline solutions, cresol red is present as a hydrophilic dianion. The dianion is small and is violet in color, possessing a larger conjugated system than the yellow hydroxyquinone. Application Cresol Red is a widely used pH indicator for routine pH assessments like microbial culture media preparations. In addition, Cresol red is a suitable constituent of PCR reagent systems. Cresol Red is used as a tracking dye in DNA, RNA (agarose), and protein (polyacrylamide) electrophoresis.

CoomassiebrilliantblueR250, Coomassie Brilliant blue R 250 (C.I. 42660), protein stain, C45H44N3NaO7S2 CAS NO.6104-59-2 MW825.97 UNSPSC Code:41105305 EC Index Number:228-060-5, potency >5000 mg/kg LD50, oral (Rat), technique(s) protein staining: suitable, pH 6.2 (25 °C, 10 g/L in H2O), mp174-180 °C, solubility 20 g/L, bulk density 250 kg/m3, εmax ≥300 at 554-563 nm at 0.025 g/L (pH 7.0), application(s) diagnostic assay manufacturing hematology histology, storage temp. 2-30°C, Identity (UV/VIS-Spectrum): passes test, Absorption maximum λmax. (buffer pH 7.0): 554 - 563 nm, Spec. Absorptivity A 1%/1cm (λmax; 0.025g/l; buffer PH7.0; calc. on dried substance): ≥ 300, TLC-Test: passes test, Loss on drying (110 °C): ≤ 5 %, Suitability for electrophoresis: passes test

CoomassiebrilliantblueG250, Coomassie Brilliant blue G 250 (C.I. 42655), Acid blue 90, Brilliant Blue G, Cyanine G, Polar Blue G, SERVA BLUE G, protein gel stain, C47H48N3NaO7S2 CAS NO.6104-58-1 MW854.02 UNSPSC Code:41105305 EC Index Number:228-058-4, potency >5000 mg/kg LD50, oral (Rat), technique(s) protein staining: suitable, pH6.4 (20 °C, 10 g/L in H2O), solubility 40 g/L, bulk density 520 kg/m3, storage temp.no temp limit, Coomassie Brilliant blue G 250 (C.I. 42655) is a member of Coomassie brilliant blue (CBB), which is a triphenyl methane dye. CBB dyes are widely used for the visualization of proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. CBB G-250 (greenish tinted blue) dye is preferably used in Bradford assay for the quantification of proteins. Application Coomassie Brilliant blue G 250 (C.I. 42655) has been used to stain gel in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. It has also been used in Bradford protein assay. Analysis Note Identity (UV/VIS-Spectrum): passes test, Absorption maximum λmax. (buffer pH 7.0): 577 - 584 nm, Spec. Absorptivity A 1%/1cm (λmax; 0.01g/l; buffer pH7.0; calc. on dried substance): 450 - 570, TLC-Test: passes test, Loss on drying (110°C): ≤ 8 %, Suitability for electrophoresis: passes test

MTT, Thiazolyl Blue Tetrazolium Bromide, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, Methylthiazolyldiphenyl-tetrazolium bromide, C18H16BrN5S CAS NO.298-93-1 MW414.32 Beilstein:4081397 EC No.206-069-5 UNSPSC Code:12352111, Assay 98%, form powder, technique(s) titration: suitable, color dark yellow, mp195 °C (dec.) (lit.), solubility 2-methoxyethanol: soluble 20 mg/mL, ethanol: soluble 20 mg/mL, H2O: 5 mg/mL, application(s) diagnostic assay manufacturing hematology histology, storage temp.2-8°C, Application Thiazolyl Blue Tetrazolium Blue (MTT) may be used in measurement of cell proliferation. MTT produces a yellowish solution that is converted to dark blue, water-insoluble MTT formazan by mitochondrial dehydrogenases of living cells. The blue crystals are solubilized with acidified isopropanol and the intensity is measured colorimetrically at 570 nm. Thiazolyl Blue Tetrazolium Blue (MTT) may be used in measurement of cell proliferation. MTT produces a yellowish solution that is converted to dark blue, water-insoluble MTT formazan by mitochondrial dehydrogenases of living cells. The blue crystals are solubilized with acidified isopropanol and the intensity is measured colorimetrically at 570 nm. MTT has been used as a histochemical/cytochemical reagent and for the detection of NAD. ADP-linked enzyme systems in tissue cannot be detected with MTT, due to binding of the cation by the cyanide trap used. MTT is rapidly reduced to the formazan, which chelates with nickel, copper, and cobalt; the cobalt chelate has been used in oxidative systems. Thiazolyl Blue Tetrazolium Bromide has been used for the following assays: Proliferation assay. TNF (tumor necrosis factor) bioassay.

Trypan Blue, Direct blue 14, Niagara blue reagent, blue live/dead cell assay C34H24N6O14S4Na4 CAS NO.72-57-1 MW960.81 Colour Index Number:23850 Beilstein:4360496 EC No.200-786-7 UNSPSC Code:12352207, form powder, color Brown to Very Dark Brown and Black-Brown dark green, composition Dye content, 70%, storage condition dry at room temperature, technique(s) cell culture | mammalian: suitable, tissue processing: suitable, mp>300 °C (lit.), solubility H2O: 10 mg/mL, application(s) cell analysis, General description The trypan blue dye is an azo dye, derived from toluidine. It is soluble in water and has a molecular weight of 961 g/mol. Application Trypan blue has been used: in cell viability assay, to stain plant tissue in order to to visualize cell death, Biochem/physiol Actions The trypan blue dye is useful to differentiate the dead and live cells based on the cell′s ability to take up or exclude the dye. A viable cell is assumed to possess an intact cell membrane and hence excludes this dye and the cytoplasm appears clear. While on the other hand non-viable cells shows a blue cytoplasm. Preparation Note To make a cell viability solution, add the T6146 Trypan Blue powder to a clean and dry glass or plastic container of appropriate capacity. Add a suitable solvent, such as sterile distilled water, to the container with the Trypan Blue powder. Stir the solution vigorously using a stir bar or a glass rod until the powder has completely dissolved. A 0.4% solution is a common concentration for typical cell viability assessment. For example, to make 100 mL of a 0.4% solution, add 400 milligrams (0.4 g) to 99.6 mL solvent.

Methylene Blue hydrate 3,7-Bis(dimethylamino)phenothiazin-5-ium chloride xhydrate, Methylene Blue monohydrate, Methylene blue, Nucleic acid staining reagent, C16H18ClN3S·xH2O CAS NO.122965-43-9 MW319.85 (anhydrous basis) Beilstein:3599847 EC No.200-515-2 UNSPSC Code:12171500, assay 98%, form Fine Crystalline Powder, color Green, technique(s) nucleic acid detection: suitable, solubility water: 1 mg/mL (dark blue), suitability suitable for nucleic acid staining, storage temp.room temp, General description Methylene blue is a heterocyclic aromatic chemical, appearing as a dark green solid powder, changing to blue in aqueous solution. It is used as a stain in many different histology procedures, as well as in molecular biology laboratories. Application Methylene Blue hydrate is suitable for use as a nucleic acid stain for agarose and polyacrylamide gel electrophoresis (PAGE). It was used to stain 4T1 tumor cells to study metastasis of tumor cells using clonogenic assay. Methylene blue hydrate crystals were encapsulated in apoferritin nanocages for photodynamic therapy against tumor cells. Features and Benefits Less hazardous than staining with ethidium bromide | Viewable in visible light | Fast and easy to use

TCEP, Tris(2-carboxyethyl)phosphine hydrochloride, C9H15O6P·HCl CAS NO.51805-45-9 MW286.65 Beilstein:3724376 UNSPSC Code:12352128, Assay ≥98% (NMR), form crystals, color white to yellow, reaction suitability, reagent type: reductant, loss ≤1.0% loss on drying, pH1.2-1.5 solubility H2O: 1 M, clear, colorless (no residue), anion traces sulfate (SO42-): ≤50 mg/kg cation traces Al: 5 mg/kg As: ≤0.05 mg/kg Ba: ≤5 mg/kg Bi: ≤5 mg/kg Ca: ≤10 mg/kg Cd: ≤5 mg/kg Co: ≤5 mg/kg Cr: ≤5 mg/kg Cu: ≤5 mg/kg Fe: ≤5 mg/kg K: ≤50 mg/kg Li: ≤5 mg/kg Mg: ≤5 mg/kg Mn: ≤5 mg/kg Mo: ≤5 mg/kg Na: ≤50 mg/kg Ni: ≤5 mg/kg Pb: ≤5 mg/kg Sr: ≤5 mg/kg Zn: ≤5 mg/kg, λ0.1 M in H2O, UV absorption λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.03, storage temp. 2-8°C, General description Tris(2-carboxyethyl)phosphine hydrochloride (TCEP.HCl) is a non-volatile solid. It is a strong reducing agent. It can be synthesized by the acid hydrolysis of tris(2-cyanoethyl)phosphine in refluxing aqueous HCl. It has various biological applications such as in vitro and in vivo reduction of disulfide bonds in various peptides and proteins. TCEP is a useful chelating agent for various heavy metal ions as Zn(II), Cd(II), Pb(II), and Ni(II). Application Tris(2-carboxyethyl)phosphine hydrochloride (TCEP. HCl) can be used: As a reducing agent for the reduction of sulfoxides, sulfonyl chlorides, N-oxides, and azides. It can also be used in azide-alkyne cycloaddition reaction in the presence of a copper catalyst. To reduce disulfide bonds in various proteins. As a reagent for the selective reduction of disulfides in water. To remove ruthenium-derived metathesis catalysts via aqueous washing of a crude reaction mixture when it is basified. As a reducing agent for the reduction of various alkyl disulfides such as trans-4,5-dihydroxy-1,2-dithiane. Water soluble reagent, used for selective reduction of disulfides. More stable than DTT and useful in mass spectrometry applications.

ABTS, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, Diammonium 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) C18H24N6O6S4 CAS NO.30931-67-0 MW548.68 Beilstein:7329461 EC No.250-396-6 UNSPSC Code:12352204, Assay ≥98% (HPLC), form powder, color Light green to green, solubility water: 10 mg/mL, clear to slightly hazy, storage temp.2-8°C, General description ABTS, 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), is water soluble chemical compound used as chromogenic substrate: ABTS together with hydrogen peroxide for reaction with peroxidase enzymes (like HRP for example). ABTS together with laccase or bilirubin oxidase. ABTS mainly used in ELISA (enzyme-linked immunosorbent assay) procedures. A peroxidase reaction of ABTS in the presence of hydrogen peroxide produces a green soluble end product which can be read spectrophotometrically at 405nm. The reaction may be stopped with 1% sodium dodecyl sulfate (SDS).††† ABTS, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt is light sensitive. Application 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) is a peroxidase substrate suitable for use in ELISA procedures. This substrate produces a soluble end product that is green in color and can be read spectrophotometrically at 405 nm. The reaction may be stopped with 1% sodium dodecyl sulfate (SDS). Recommended for ELISA (microwell) procedures, not recommended for membrane applications. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt has been used as a substrate in ELISA (enzyme-linked immunosorbent assay). ABTS is significantly used by the food industry, to measure the antioxidant capacities within different foods. ABTS is also used to monitor glucose concentrations in different solutions including blood serum. It is recommended for ELISA (microwell) procedures, not recommended for membrane applications.Since it is less readily oxidized than TMB and OPD substrates, ABTS is less sensitive for ELISA applications. Thus ABTS has some advantageous over TMB and OPD in cases of large background results.

TMB, 3,3',5,5'-tetramethylbenzidine, BM blue, Sure Blue TMB, TMB, TMB Blotting Plus, TMB substrate, [-C6H2(CH3)2-4-NH2]2, CAS NO.54827-17-7 MW240.34 Beilstein:2808541 EC No.259-364-6 UNSPSC Code:12352204, Assay ≥98% (TLC), form powder, color white to light yellow, mp168-171 °C (lit.), solubility ethyl acetate: 50 mg/mL, clear to very slightly hazy, storage temp.2-8°C, Application 3,3′,5,5′-Tetramethylbenzidine (TMB) is a noncarcinogenic substitute (Ames test negative) for benzidine suitable as peroxidase substrate for use in ELISA procedures. The substrate produces a soluble end product that is pale blue in color and can be read spectrophotometrically at 370 or 620-650 nm. The TMB reaction may be stopped with 2 M H2SO4 (resulting in a yellow color), and read at 450 nm. A sensitive and specific reagent for the detection of blood, assay of hemoglobin, assay of peroxidases. 3,3′,5,5′-Tetramethylbenzidine has been used:as a supplement in Man-Rodosa-Sharpe (MRS) agar plate, to stains the lactobacilli colonies blue in the presence of O2, as a substrate solution for ELISA (enzyme-linked immunosorbent assay), in myeloperoxidase activity assay to quantify neutrophil. Biochem/physiol Actions 3,3′,5,5′-Tetramethylbenzidine/TMB can be used as a chromogen to increase the progression of product obtained in peroxidase reaction. In food and environmental decontamination procedures, TMB can be used in in situ free available chlorine (FAC) monitoring of chlorite-based sanitizers.

AMPPD, 3-(2'-Spiroadamantane)-4-methoxy-4-(3'-phosphoryloxy)phenyl-1,2-dioxetane, C18H23O7P CAS NO.122341-56-4 MW382.345 form crystalline powder, color white, assay 95%, mp548.6±60.0 °C at 760 mmHg, bp548.6 °C at 760 mmHg, solubility water:slightly soluble,

3-Aminophthalhydrazide, Luminol, 5-Amino-2,3-dihydro-1,4-phthalazinedione, C8H7N3O2 CAS NO.521-31-3 MW177.16 Beilstein:383929 EC No.208-309-4 UNSPSC Code:12352204, assay 98%(HPLC), form powder or chunks, color yellow to green, mp330.5 °C (lit.), solubility DMSO: 50 mg/mL, clear to slightly hazy, General description Luminol is a chemiluminescent compound, which belongs to the class of cyclic acyl hydrazides. It exhibits chemiluminescence, when oxidized by oxidizing agents. Luminol may be associated with nausea, vomiting and mucosa irritation in eyes and skin. Application Luminol has been used in chemiluminescence assay. Features and Benefits Used for chemiluminescence analysis of, for example, metal cations, blood, and glucococorticoids.
TTC, TPTZ, TTZ, 2,3,5-Triphenyl-2H-tetrazolium chloride, Tetrazolium Red, C19H15ClN4 CAS NO.298-96-4 MW334.80 Beilstein:3923356 EC No.206-071-6 UNSPSC Code:12171500, Assay ≥98.0% (HPLC), form powder, color faintly yellow, solubility H2O: 50 mg/mL, application(s) diagnostic assay manufacturing | hematology | histology, storage temp.2-8°C, General description 2,3,5-Triphenyltetrazolium chloride is a colorless water-soluble dye. In the mitochondria of living cells, it is reduced to a deep red, water-insoluble compound (formazan). 2,3,5-Triphenyltetrazolium chloride helps to distinguish between viable and infarcted brain tissue after stroke. Application 2,3,5-Triphenyltetrazolium chloride has been used to stain heart tissue to measure the extent of acute lesion.[2] It has also been used to stain brain tissue to check the size of infarct area.

NBT,Nitrotetrazolium Blue chloride, 2,2′-bis(4-Nitrophenyl)-5,5′-diphenyl-3,3′-(3,3′-dimethoxy-4,4′-diphenylene)ditetrazolium chloride, 3,3′-(3,3′-Dimethoxy-4,4′-biphenylene)bis[2-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride], p-Nitro-Blue tetrazolium chloride, p-Nitrotetrazolium blue, Nitro BT, C40H30N10O6·2Cl CAS NO.298-83-9 MW817.64 Beilstein:4115923 UNSPSC Code:41116100, assay 98%, mp189°C, form crystals powder, color yellow, solubility water: soluble, General description NBT is soluble in water, aqueous buffers, and dimethylformamide. Tetrazolium salts are useful in determining the metabolic activity of cells from many origins. The quaternary tetrazole ring core containing four nitrogen atoms, with a net positive charge induces cellular uptake through plasma membrane potential. This drives its use in cell biology. In immunolabeling technique, 4-Nitro blue tetrazolium (NBT) serves as an oxidant. The hydrolysis of 5-bromo-4-chloro-3-indolyl phosphate (BCIP) by alkaline phosphatase gives rise to leucoindigo, which is then being reduced by NBT to form an insoluble blue BCI. Certain cells reduce NBT, which is a colorless/yellow dye to blue or black formazan crystals. Due to this property, NBT is prefered in redox histochemistry and in biochemical applications. It is formerly used to distinguish between nonbacterial and bacterial diseases, the latter causing neutrophils to reduce the dye; used to confirm diagnosis of chronic granulomatous disease. Application Colony and plaque hybridization Immunohistocytochemistry In situ hybridization Northern blot Southern blot Western blot NBT has been used in in situ hybridization. Preparation Note Working solution: Solubility: soluble in water, aqueous buffers, and dimethylformamide. Can be dissolved in methanol. A clear solution is achieved at 20 mg/ml. Analysis Note Absorption: NBT has its maximum absorption at 260 nm [in water], and the final product formazan has its maximum absorption at 605 nm [in nitrobenzole]. Performance-controlled in the LDH assay. Other Notes For life science research only. Not for use in diagnostic procedures.

DTNB, 5,5'-Dithiobis(2-nitrobenzoic acid), 3-Carboxy-4-nitrophenyl disulfide, 6,6′-Dinitro-3,3′-dithiodibenzoic acid, Bis(3-carboxy-4-nitrophenyl) disulfide, DTNB, Ellman’s Reagent, 5,5′-Dithio-bis-(2-nitrobenzoic Acid), [-SC6H3(NO2)CO2H]2 CAS NO.69-78-3 MW396.35 Beilstein:1896821 EC No.200-714-4 UNSPSC Code:12352106, Assay ≥98%, form powder, color yellow, mp240-245 °C (dec.) (lit.), suitability suitable for determination of sulfhydryl groups, General description 5,5′-Dithiobis(2-nitrobenzoic acid) is also called DTNB or Ellman′s reagent. It is a water soluble reagent, which interacts with thiol groups, and can be used conveniently at neutral pH. Thiolate anion (RS-) reacts with Ellman′s reagent (DTNB2-), to produce 2-nitro-5-thiobenzoate anion (TNB2-) and one mixed disulfide (R-S-TNB-). TNB2- has a high molar absorption coefficient, and absorbs at 412nm. This absorption is independent of pH, at pH> 7.3. Application Ellman′s reagent maybe used for acetylcholinesterase assay, and to specifically label cysteine residues. It is a sensitive reagent for measuring the free sulfhydryl content in proteins, peptides, and tissues.

Guanidine hydrochloride, Aminoformamidine hydrochloride, Aminomethanamidine hydrochloride, Guanidinium chloride, NH2C(=NH)NH2·HCl CAS NO.50-01-1 MW95.53 Beilstein:3591990 EC No.200-002-3 UNSPSC Code:12352107, Assay ≥99%, form crystalline powder, color white to slightly yellow, storage condition (Tightly closed. Dry. ), technique(s) RNA extraction: suitable, impurities ≤0.3% water (Karl Fischer), pH(25 °C, 4.6 - 6/573 g/L), mp180-185 °C (lit.), solubility H2O: 6 M, clear, colorless, density 1.3 g/cm3 (lit.), cation traces Pb: ≤5 ppm, UV absorption λ: 260 nm Amax: ≤0.10 λ: 290 nm Amax: ≤0.05, foreign activity DNase, Nase, none detected, General description Guanidine hydrochloride, also known as aminoformamidine hydrochloride, is a versatile compound widely used in molecular biology and biochemical research. Functioning as a potent chaotropic agent, it disrupts protein and nucleic acid structures, making it invaluable for purifying proteins, particularly mRNA. Its denaturing capabilities extend to unfolding proteins, aiding their purification and subsequent refolding, and it can even solubilize denatured insoluble proteins at higher concentrations. At lower concentrations, guanidine hydrochloride exhibits a unique effect, promoting the refolding of denatured proteins and restoring enzymatic activity. This property is advantageous for protein renaturation studies. In RNA extraction, guanidine hydrochloride acts as a robust denaturant, disrupting cell structures and inactivating RNA enzymes, ensuring the integrity of extracted RNA. In summary, guanidine hydrochloride is a versatile compound with applications in protein purification, nucleic acid isolation, and protein refolding studies, showcasing its multifaceted utility in molecular biology and biochemical research. Application Guanidine hydrochloride has been used: as a component of the extraction buffer for the extraction of proteoglycans, in extraction during protein fractionation of ATDC5 cell lines as a chemical additive to study its effective absorbance spectra in structural analysis to incubate the device chip to release prostate-specific antigen and for regeneration of aptamer as a component of the 2,4-dinitrophenylhydrazine (DNPH) solution for detecting protein carbonyls by protein carbonylation study in RNA isolation to dissociate nucleoproteins and inhibit RNase Strong chaotropic agent useful for the denaturation and subsequent refolding of proteins. This strong denaturant can solubilize insoluble or denatured proteins such as inclusion bodies. This can be used as the first step in refolding proteins or enzymes into their active form. Urea and dithiothreitol (DTT) may also be necessary. Biochem/physiol Actions Guanidine hydrochloride (GuHCl) is a small hydroscopic molecule. It plays a role in inhibiting heat shock protein 104 (Hsp104) adenosine triphosphatase (ATPase) activity in vivo. It is a potent denaturant and inactivator of several enzymes and proteins. GuHCl can inactivate aminoacylase and papain. It is involved in unfolding and inactivation of alkaline phosphatase in Haliotis diversicolor. At higher concentrations, GuHCl is involved in virus inactivation such as Herpes simplex virus 1 (HSV-1). Features and Benefits Highly versatile surfactant for your cell biology and biochemical research Suitable for sensitive molecular biology applications Free from Exonuclease, Dnase and Rnase Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications. Caution May agglomerate upon storage. The quality of the product does not appear to be affected and solutions prepared from the free-flowing and lumpy guanidine hydrochloride appear identical. Preparation Note In order to make an 8M solution in water, one must heat the solution to 35 °C for approximately 30 minutes. The maximum solubility of guanidine hydrochloride in water at room temperature is approximately 6M.

Guanidine thiocyanate, Guanidinium rhodanide, Guanidinium thiocyanate, NH2C(=NH)NH2·HSCN CAS NO.593-84-0 MW118.16 Beilstein:3563461 EC No.209-812-1 UNSPSC Code:12352107, grade Molecular Biology, Assay ≥99%, form powder or crystals, storage condition (Tightly closed. Dry. ), technique(s) RNA extraction: suitable, color colorless to white, mp120-122 °C (lit.), solubility water: 100 mg/mL, clear, colorless, density1.29 g/cm3 at 20 °C (68 °F), cation traces Ba: ≤5 ppm Fe: ≤5 ppm Na: ≤0.5%, General description Guanidine thiocyanate, or guanidinium rhodanide, is a versatile chemical compound widely used in molecular biology and biochemical research. Acting as a chaotropic agent, it disrupts cell and organelle membranes, facilitating cell lysis and organelle isolation. Notably, it serves as a potent protein denaturant, surpassing guanidine hydrochloride in unfolding proteins. This property is valuable for purifying nucleic acids from crude cell lysates, effectively separating nucleic acids from denatured proteins. In nucleic acid purification, guanidine thiocyanate is effective in inactivating DNases and RNases, preventing nucleic acid degradation and ensuring favorable yields. Its dual denaturing and enzyme-inhibiting properties make it crucial for DNA and RNA studies. Beyond nucleic acid isolation, guanidine thiocyanate is used for cell lysis and virus particle inactivation during RNA and DNA extractions. It not only disrupts membranes but also denatures RNase and DNase enzymes, protecting extracted nucleic acids from enzymatic degradation. The versatility of guanidine thiocyanate extends to molecular biology techniques like PCR, Northern blotting, and Southern blotting. It serves as a vital reagent in these methods, contributing to the successful analysis and amplification of nucleic acids. Application Chaotropic agent and strong denaturant; solubilizes cells. Guanidine thiocyanate has been used: for the lysis of colorectal adenocarcinoma cell line prior to RNA isolation as a PCR inhibitor in triplex mitochondrial DNA (mtDNA) qPCR assay for DNA extraction from cerebrospinal fluid (CSF). Biochem/physiol Actions Guanidine thiocyanate is a denaturing agent and is used routinely in RNA isolation. It is used as a storage buffer for whole blood samples. Guanidine thiocyanate inactivates nucleases and is ideal for storing and freezing fecal samples for DNA studies. It is used in combination with phenol−chloroform in RNA extraction. Features and Benefits Highly versatile surfactant for your cell biology and biochemical research Suitable for RNA extraction in sensitive molecular biology applications Free from Dnase and Rnase Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications

BSA, Albumin bovine serum, BSA, Bovine albumin, CAS NO.9048-46-8 EC No.232-936-2 UNSPSC Code:12352202, biological source bovine, product line BioReagent, Assay ≥98%, 96%, form lyophilized powder, mol wt ~66 kDa, purified by chromatography, packaging poly bottle of, technique(s) cell culture | mammalian: suitable, impurities ≤1 EU/mg endotoxin, pH7, solubility water: soluble (40 mg/ml), General description Bovine serum albumin (BSA) is a 66 kDa globular protein crucial for transporting molecules like fatty acids, drugs and hormones from the bloodstream. BSA is a drug carrier for bioactive compounds and has wide clinical applications. BSA has extensive usage in biological research including cell culture, molecular biology, protein biochemistry, and detection techniques. It has wide pharmaceutical applications due to ease of production and interaction flexibility. Application Bovine serum albumin has been used: as a standard in bradford protein estimation method in yeast cells, in elispot assay of blood cells, as a blocking agent in immunohistochemical studies of transplant, as a blocking agent in western blotting of human gastric cancer cell lines. Biochem/physiol Actions Certain conformational and primary-sequence epitopes of BSA are suspected allergens in human beef and milk allergies. Features and Benefits Tested for use in cell culture Low endotoxin. Preparation Note Prepared by salt fractionation, ion exchange, and gel filtration chromatography. Serum albumin may be referred to as Fraction V. This naming convention is taken from the original Cohn method of fractionating serum proteins using cold ethanol precipitation. Serum albumin was found in the fifth ethanol fraction using Cohn′s method. Since then, the term "Fraction V" has been used by some to describe serum albumin regardless of the method of preparation. Others have used this term to describe serum albumin purified by ethanol fractionation methods that have been highly modified since the original Cohn method was described. Sigma-Aldrich manufactures and distributes serum albumins purified from a variety of primary methods including the true Cohn fractionation method, modified ethanol fractionation methods, heat shock and chromatography. Additional purification steps may include crystallization or charcoal filtration.

Human serum albumin, CAS NO.70024-90-7 EC No.274-272-6 UNSPSC Code:12352202, biological source human, Assay ≥99% (agarose gel electrophoresis), form lyophilized powder, mol wt monomer calculated mol wt 66478 Da, technique(s) ELISA: suitable, tissue culture: suitable, western blot: suitable, impurities HIV I and HIVII, HCV and HBsAg, tested negative ≤0.1% Fatty acids, solubility H2O: soluble 50 mg/mL, UniProt accession no.P02768, storage temp.2-8°C, General description Albumin is the most copious protein in blood plasma. Liver produces human albumin. Human albumin possess a molecular weight of 67 kDa. Application Albumin from human serum has been used: to standardize the column for molecular-exclusion chromatography, to prepare the tobacco mosaic virus (TMV)-poly ethylene glycol (PEG8)-serum albumin (SA) particles, to analyze its modifications after oxidation using a Fenton system, Albumin-bound fluorescence was used in serum of patients with chronic renal failure. Biochem/physiol Actions Albumin turnover is seen in infants with iron deficiency anemia. Serum albumin is a reliable prognostic indicator in liver disease. Oxidative modification of albumin is seen in advanced liver disease. Albumins are soluble monomeric proteins found in the body fluids and tissues of animals and in some plant seeds. Serum albumin functions as a carrier protein for steroids, fatty acids, and thyroid hormones. Serum albumins are also vital in regulating the colloidal osmotic pressures of blood. Serum albumin functions as a carrier protein for steroids, fatty acids, and thyroid hormones, and is vital in regulating the colloidal osmotic pressures of blood. Albumin is also seen to bind to exogenous substances, particularly drugs (e.g., ibuprofen, warfarin), and strongly influence their pharmacokinetics. Oxidative stress leading to changes in the redox state of albumin has widely varied effects on its physiological function. Preparation Note Prepared using method IV of Cohn, E.J., et al., J. Am. Chem. Soc., 69, 1753 (1947).

Proteinase K, Endopeptidase K, C29H27N2O12P CAS NO.39450-01-6 MW626.50 EC No.3.4.21.64 (BRENDA, IUBMB) EC No.254-457-8 UNSPSC Code:12352204, form lyophilized powder, color white, specific activity ≥30 units/mg protein, mol wt 28.93 kDa, technique(s) DNA extraction: suitable, solubility H2O: soluble 1 mg/mL, clear, colorless, foreign activity Dnase ≤30 Kunitz units/mg solid RNase ≤0.003 Kunitz units/mg solid, shipped in wet ice, storage temp.−20°C. Application Useful for the proteolytic inactivation of nucleases during the isolation of DNA and RNA. Useful for the proteolytic inactivation of nucleases during the isolation of DNA and RNA. Removes endotoxins that bind to cationic proteins such as lysozyme and ribonuclease A. Reported useful for the isolation of hepatic, yeast, and mung bean mitochondria Determination of enzyme localization on membranes. Treatment of paraffin embedded tissue sections to expose antigen binding sites for antibody labeling. Digestion of proteins from brain tissue samples for prions in Transmissible Spongiform Encephalopathies (TSE) research. Biochem/physiol Actions Proteinase K is a stable and highly reactive serine protease. Evidence from crystal and molecular structure studies indicates the enzyme belongs to the subtilisin family with an active-site catalytic triad (Asp39-His69-Ser224). It is stable in a broad range of environments: pH, buffer salts, detergents (SDS), and temperature. In the presence of 0.1-0.5% SDS, proteinase K retains activity and will digest a variety of proteins and nucleases in DNA preparations without compromising the integrity of the isolated DNA. Analysis Note Appearance (color): white, Appearance (description): lyophilisate, Activity (hemoglobin; pH7.5; 37°C): ≥ 30.0 mAnsonU/mg, Spec. activity (calc. on protein): ≥ 40 mAnsonU/mg, DNases (Nicking activity; pBR 322; 6h; 37°C): not detectable, RNases (RNA; 2 h; 37°C): not detectable

SDS, Dodecyl sodium sulfate, Dodecyl sulfate sodium salt, Lauryl sulfate sodium salt, SDS, Sodium lauryl sulfate, Sodium dodecyl sulfate, Sodium n-Dodecyl Sulfate, Sodium n-Dodecyl Sulfate, Molecular Biology Grade, Dodecyl hydrogen sulfate sodium salt, Sodium n-Dodecyl Sulfate, High Purity, SDS Tablets, CH3(CH2)11OSO3Na CAS NO.151-21-3 MW288.38 Beilstein:3599286 EC No.205-788-1 UNSPSC Code:12161902 grade Molecular Biology, description anionic product line BioReagentm Assay ≥98.5% (GC) form powderm, mol wt micellar 18,000, aggregation number 62, technique(s) electrophoresis: suitable, impurities ≤2.0% water (Karl Fischer), CMC 7-10 mM (20-25°C), mp204-207 °C (lit.), transition temp cloud point >100 °C, solubility water: soluble, density 1.03 g/cm3 at 20 °C (68 °F), anion traces chloride (Cl-): ≤500 ppm phosphate (PO43-): ≤10 ppm cation traces heavy metals (as Pb): ≤10 ppm, absorption ≤0.1 at 260 at 3% ≤0.1 at 280 at 3%, HLB 40, suitability suitable for electrophoresis, foreign activity DNase, RNase, none detected, storage temp.room temp, General description Sodium dodecyl sulfate (SDS), also known as sodium lauryl sulfate, is an anionic surfactant widely used in various scientific applications, particularly in molecular biology, biochemical, and cell biology research. Its effectiveness stems from its amphiphilic nature, allowing it to interact with both hydrophobic and hydrophilic molecules. SDS is a versatile detergent that excels in solubilizing and denaturing proteins, making it an essential tool in protein extraction and purification procedures. By disrupting protein structures, SDS facilitates the separation of proteins based on their molecular weight during SDS-PAGE electrophoresis. Beyond protein analysis, SDS also finds applications in the electrophoretic separation of lipids, enabling the study and characterization of these important cellular components. Additionally, SDS is employed in various hybridization and molecular biology techniques, demonstrating its versatility across a range of research areas. SDS′s effectiveness in both ionic and anionic conditions further expands its utility, allowing it to adapt to diverse experimental setups and requirements. Its ability to unravel proteins by binding to peptide chains and imparting a negative charge effectively eliminates protein shape as a factor for gel separation, ensuring accurate protein size determination. Application Anionic detergent Sodium dodecyl sulfate has been used: in chromatin immunoprecipitation, in SDS-polyacrylamide gel electrophoresis (SDS-PAGE), as a component of radioimmunoprecipitation assay buffer for immunoblotting, Used to solubilize and denature proteins for denaturing-PAGE. Most proteins bind SDS in a ratio of 1.4 g SDS per gram of protein. The charges intrinsic to the protein become insignificant compared to the overall negative charge provided by the bound SDS. The charge to mass ratio is essentially the same for each protein and will migrate in the gel based only on their size. Features and Benefits Highly versatile surfactant for your molecular biology and biochemical research, Suitable for electrophoresis applications, Tested to confirm low levels of heavy metal contamination,ensuring suitability for various applications. Analysis Note Tested for use in denatured polyacrylamide gel electrophoresis.

Sodium N-lauroyl sarcosinate, N-Lauroylsarcosine sodium salt, N-Dodecanoyl-N-methylglycine sodium salt, Sarkosyl NL, CH3(CH2)10CON(CH3)CH2COONa CAS NO.137-16-6 MW293.38 EC No.:205-281-5 Beilstein No.:5322974, 10% solution, assay 98%(HPLC), form powder, color white, mol wt micellar avg mol wt 600, aggregation number 2, technique(s) protein expression: suitable protein purification: suitable protein quantification: suitable, impurities ≤7% water, CMC 14.6 mM (20-25°C), mp46 °C(lit.), solubility H2O: very soluble 293 g/L at 20 °C, General description N-Lauroylsarcosine is an anionic surfactant with an ability to denature proteins. Due to its microbicidal property, N-lauroylsarcosine is being considered as a potent anti-microbicide in topical formulations, especially against sexually transmitted diseases (STDs). Application N-Lauroylsarcosine sodium salt has been used to study the complexing ability of a novel amphiphilic γ-cyclodextrin with anionic surfactants. Other Notes Anionic surfactant.

TMTU; 1,1,3,3-Tetramethylthiourea CAS NO.2782-91-4 C5H12N2S MW132.23 pure 25g
1-(2-Hydroxyethyl)piperazine; HEP CAS NO.103-76-4 C6H14N2O MW130.19
o-Sulfobenzoic acid ammonium salt CAS NO.6939-89-5 C7H9NO5S MW219.2151 pure 25g
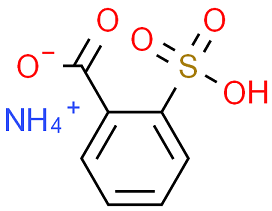
2-(4-aminophenyl)-6-methylbenzothiazole CAS NO.92-36-4 C14H12N2S MW240.32 pure 25g
-6-methylbenzothiazole.png)
4:4'-Di(6-methylbenzthiazyl-2)azobenzene C28H20N4S2 MW476.61736 pure 25g
azobenzene.png)
Di(chloromethyl)-4:4'-di(6-methylbenzthiazyl-2)azobenzene C30H22Cl2N4S2 MW573.56004 pure 25g
-4 4'-di(6-methylbenzthiazyl-2)azobenzene.png)
N-tosyl-L-alanine; Tos-Ala-OH CAS NO.21957-58-4 C10H13NO4S MW243.28 pure 25g
Tetrabromophthalic anhydride CAS NO.632-79-1 C8Br4O3 MW463.70 pure 25g
Phenolphthalein USP/BP CAS NO.77-09-8 pure 25g
2,3-Dibromomaleimide CAS NO.1122-10-7 C4HBr2NO2 MW254.86pure 25g
Dehydrothio-p-toluidine-o-sulfonic acid CAS NO.130-17-6 pure 25g
Dehydrothio-p-toluidinedisulfonic acid CAS NO.5855-98-1 pure 25g
Bromophenol red; 3′,3′′-Dibromophenolsulfonphthalein; 5',5''-Dibromophenolsulfonphthalein; BPR C19H12Br2O5S CAS NO.2800-80-8 MW512.17 UNSPSC Code:12171502 EC Index Number:220-538-1, form solid powder, color red to red-brown to brown, mp230 °C (decomposition), bulk density 770 kg/m3, Identity (UV/VIS-Spectrum): passes test, Transition range: pH 4.7 - pH 6.3 yellow to purple, Absorption maximum λ 1 (buffer pH4.7): 435 - 440 nm, Absorption maximum λ2 (buffer pH6.3): 574 - 577 nm, Spec. Absorptivity A 1%/1cm (λ1max; 0.01g/l; buffer pH4.7; calculated on anhydrous substance): ≥ (1)AR 380, (2)High purity 452,Spec. Absorptivity A 1%/1cm (λ2max; 0.01g/l; buffer pH6.3; calculated on anhydrous substance): ≥ (1)AR 760, (2)High purity 904,Water (according to Karl Fischer): ≤ 0.5 %, applications: Sensors, sol-gelmatrix, recordingmaterials, thermochromic materials, inks, paints, alloy coatings, lubricants, soaps, cosmetics, identifying fresh & stale rice, acidity inwine, food storage, determina Chemical booktion of bacterial growth, antiamyloid agents, evaluating dental caries activity, determination of Streptococciin human saliva, diagnosis of enterohemorrhagicEscherichiacoli, treatment of acuteleukemia, Bromophenol Red is a luminol signal co-enhancer in chemiluminescent detection of sequence-specific DNA, an inhibitor of glucose 6-phosphate dehydrogenase (G6PD). Bromophenol red has been demonstrated that it binds to lysozyme and inhibits their activity against bacterial cell walls, but it does not inhibit the polysaccharide component peptidoglycan. [1] Terron, Maria C. Verhagen, Frank J.M. Franssen, Maurice C.R. Field, Jim A. Chemosphere, 1998, vol. 36, # 6 p. 1445-1452. [2] Cohen Publ. Health Rep., 1926, vol. 41, p. 3060 [3]. Employment of bromophenol red and bovine serum albumin as luminol signal co-enhancer in chemiluminescent detection of sequence-specific DNA, Xiaoqian Yu, Yingying Sheng, Yanjun Zhao, Aiping Fan, Talanta Volume 148, 1 February 2016, Pages 264-271 [4]. Mikrochim Acta. 2022 Aug 5;189(9):316, Non-invasive detection of COVID-19 using a microfluidic-based colorimetric sensor array sensitive to urinary metabolites, Mohammad Mahdi Bordbar, Hosein Samadinia, Azarmidokht Sheini, Jasem Aboonajmi, Mohammad Javid, Hashem Sharghi, Mostafa Ghanei, Hasan Bagheri.
Chlorophenol red; 3′,3′′-Dichlorophenolsulfonphthalein CAS NO.4430-20-0 C19H12Cl2O5S MW423.27 pure 25g
Maleimide CAS NO.541-59-3 C4H3NO2 MW97.07 pure 25g
Aminomethanesulfonic acid CAS NO.13881-91-9 NH2CH2SO3H MW111.12 pure 25g
Tri(4-morpholino)phosphine oxide; Trimorpholinophosphine oxide CAS NO.4441-12-7 C12H24N3O4P MW305.31 pure 25g

ADA, N-(2-acetamido)iminodiacetic acid CAS NO.26239-55-4 C6H10N2O5 MW190.2 99.5%Na+ free 25g
Alcian blue 8GS Alcian blue 8gx, C56H68Cl4CuN16S4 CAS NO.33864-99-2 MW1298.86 Colour Index Number:74240 EC No.251-705-7 UNSPSC Code:12171500, BSC Certified and pure grade 25g, form powder, composition Dye content 50%, 82.5%, technique(s) microbe id | staining: suitable, color blue, solubility H2O: 1 mg/mL, εmax 425 at 615-630 nm in water, application(s) diagnostic assay manufacturing hematology histology, storage temp.room temp. General description Alcian Blue 8GX, also known as Ingrain Blue 1, is a basic copper phthalocyanine dye whose copper content imparts a blue color. It is tetravalent, cationic, and water-soluble in nature. It forms salt linkages with the acid groups of acid mucopolysaccharides. Application Alcian Blue 8GX has been used for the staining and precipitation of glycosaminoglycans and as a heteroglycan stain for neutral, sulfated, and phosphated mucopolysaccharides and glycosaminoglycans in tissues such as cartilage and extracellular matrices. It has been employed in the in vivo visualization of intravascular Bonghan ducts and corpuscles in murine models. It has been used to develop a novel histochemical staining method to assess multiple respiratory parameters in various species. Features and Benefits Desired staining patterns can be obtained by manipulating pH and salt concentrations. Principle The Alcian blue staining is easily combined with the Periodic acid-Schiff (PAS) reaction. In the tissue, acid mucosubstances are stained selectively and nuclear fast red is commonly used as counterstain. In combination with the PAS reaction, non-substituted polysaccharides, neutral mucopolysaccharides, muco- and glycoproteins, and glycol- and phospholipids can be visualized in addition. An Acetic acid-Alcian blue solution is used for the staining, whereby carboxylated and sulfatic proteoglycans are selectively stained when a pH of 2.5 is applied. The differentiation between carboxyl and sulfate groups is only possible at pH 1. Its electrophoresis and biological staining are used to detect glycoproteins. In biological staining it is used in Mowry' pH 2.5 method, Scott's pH 5.7 method, and Kreyberg's method to detect keratin and mucus. [1]. Candiano G, et al. Widening and diversifying the proteome capture by combinatorial peptide ligand libraries via Alcian Blue dye binding. Anal Chem. 2015;87(9):4814-4820. [2]. D P Penney, et al. Analysis and testing of biological stains--the Biological Stain Commission Procedures. Biotech Histochem. 2002 Sep-Nov;77(5-6):237-75. [3]. Daisuke Matsumaru, et al. Genetic analysis of Hedgehog signaling in ventral body wall development and the onset of omphalocele formation. PLoS One. 2011 Jan 20;6(1):e16260. [4]. Dongmei Sun, et al. Constitutive L-Sox5 overexpression delays differentiation of ATDC5 cells into chondrocytes and correlates with reduced expression of differentiation markers. Mol Cell Biochem. 2015 Mar;401(1-2):21-6. [5]. Koji Mizuhashi, et al. Filamin-interacting proteins, Cfm1 and Cfm2, are essential for the formation of cartilaginous skeletal elements. Hum Mol Genet. 2014 Jun 1;23(11):2953-67.
Alcian blue pyridine variant CAS NO.123439-83-8 C56H40Cl4CuN12 MW1086.36 pure 25g
Alcian green
Alcian yellow, C.I.12840, Ingrain Yellow 1, C40H46Cl2N8S4 CAS NO.61968-76-1 MW838.03 pure 25g, form Powder, color rust-colored powder, UV ( c=15mg/L in methanol; 1cm cell ) Lamda max ca. 388nm, 0.49Amin, mp167-169D.C(dec.), [1]. Leung, K., & Gibbon, K.J. A rapid staining method for Helicobacter pylori in gastric biopsies Journal of Histotechnology, June 1996, v. 19, No. 2, pp 131, [2]. JC Stockert, P Del Castillo, R Armas-Portela. Alcian yellow as a fluorescent dye. Acta histochemica. 1989;85(1):59-63
Alcian yellow pyridine variant pure 25g
Neutral red CAS NO.553-24-2 pure 25g

Phenolphthalein monophosphate bis(cyclohexylammonium) salt CAS NO.14815-59-9 C20H15O7P·2C6H13N MW596.65 pure 25g
 salt.png)
Phenolphthalein monophosphate dipotassium salt pure 25g
Phenolphthalein monophosphate disodium salt CAS NO.108321-15-9 pure 25g
Phenolphthalein monophosphate magnesium salt pure 25g

Phenolphthalein disulfate tripotassium salt CAS NO.62625-16-5 C20H19K3O14S2 MW664.7517 pure 25g
Phenolphthalein bisphosphate tetrasodium salt CAS NO.68807-90-9 C20H12O10P2Na4 MW566.21 pure 25g
Sulfobromophthalein disodium salt hydrate; BSP; Bromsulfalein disodium salt hydrate CAS No.123359-42-2 C20H8Br4Na2O10S2.xH2O MW838.00(anhydrous basis) pure 25g,form powder,color white to light gray, composition Water (by Karl Fischer): ≤ 8%, Sodium (Na) anhydrous: 4.6-5.8%, technique(s) titration: suitable, solubility water: 50 mg/mL, clear to hazy, λmax 575-581 nm, application(s) diagnostic assay manufacturing, hematology, histology, storage temp. room temp. Application Sulfobromophthalein disodium salt hydrate has been used as a positive control to study the interaction of 11-keto-β-boswellic acid (KBA) and 3-acetyl-11-keto-β-boswellic acid (AKBA) with organic anion transporter OATP1B3. Biochem/physiol Actions Sulfobromophthalein (BSP) is a high affinity organic ion substrate for organic anion transporting polypeptides (OATPs) and has been used as a substrate for multidrug resistance associated protein 2 (Mrp2). BSP is transported into hepatocytes by OATPs and, after conjugation to glutathione, is excreted into bile by Mrp2. It was found to inhibit the aldo-keto reductase ARK1C20. [1] Permeation of Boswellia extract in the Caco-2 model and possible interactions of its constituents KBA and AKBA with OATP1B3 and MRP2, Philip Kruger, European Journal of Pharmaceutical Sciences (2009). [2]. Organic Anion Transporting Polypeptide Mediates Organic Anion/HCO3-Exchange, Lisa M. The Journal of Biological Chemistry (1997). [3]. Substrate- and pH-specific antifolate transport mediated by organic anion-transporting polypeptide 2B1 (OATP2B1-SLCO2B1). Visentin M, Molecular Pharmaceutics (2012). [4]. Introduction of multidrug restance-associated protein 2 in liver, intestine and kidney of streptozotocin-induced diabetic rats. Xenobiotica (2012).
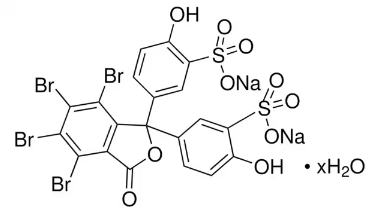
Thymolphthalein monophosphate disodium salt, TMP disodium salt, CAS NO.28749-63-5 123359-43-3 62796-27-4 C28H29Na2O7P.xH2O MW554.48(anhydrous basis), form Solid, color Off-White to Pale Beige to light yellow, Assay 99% pure anhydrous, solubility: DMSO (Slightly), Methanol (Slightly), Water (Slightly, Heated and Sonicated), Storage: 2-8DC, protect away from moisture, Applications Thymolphthalein Monophosphoric Acid Disodium Salt is a chromogenic substrate for the determination of acid phosphatase and alkaline phosphatase. It is a disodium salt form of Thymolphthalein Monophosphoric Acid which can be used as reactant/reagent in reaction of homodimer and heterodimer subunits of human prostate acid phosphatase. [1]. Lee, H., et al.: Biochem J, 277, 759 (1991). [2]. Roy AV, et al. Sodium thymolphthalein monophosphate: a new acid phosphatase substrate with greater specificity for the prostatic enzyme in serum. Clin Chem. 1971 Nov;17(11):1093-102. [3]. Morin LG. Ammonium thymolphthalein monophosphate as a new substrate for alkaline and acid phosphatase determinations in serum. Clin Chem. 1973 Oct;19(10):1135-8.
Thymolphthalein monophosphate magnesium salt CAS NO.35106-21-9 C28H29MgO7P.XH2O MW532.81(anhydrous basis) pure 25g, Substrate for use in determining the alkaline phosphatase content of blood serum and use in the determination of serum acid phosphatases, [1]. Clinica Chimica Acta, 13, pp. 401-403(1966)
o-Cresolphthalein monophosphate disodium salt pure 25g

o-Cresolphthalein monophosphate magnesium salt pure 25g

1-Naphtholphthalein monophosphate disodium salt pure 25g

1-Naphtholphthalein monophosphate magnesium salr pure 25g

3-(N-Tosyl-L-alaninyloxy) indole CAS NO.75062-54-3 pure 25g
 indole.png)
2,4-Dichlorobenzenediazonium tetrafluoroborate CAS NO.21872-70-8 C6H3Cl2N2BF4 MW189.907913 pure 25g
2-Methoxy-4-nitrobenzenediazonium tetrafluoroborate CAS NO.2357-51-9 25g
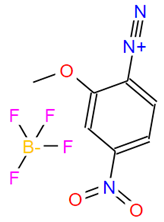
4-Bromobenzenediazonium tetrafluoroborate CAS NO.673-40-5 BrC6H4N2BF4 MW270.82 25g
2,4-Dichlorobenzenediazonium 1,5-naphthalenedisulfonate hydrate CAS NO.123333-91-5 2,4-DCPD, 2,4-Dichlorophenyldiazonium 1,5-naphthalenedisulfonate Cl2C6H3N2(O3SC10H6SO3H).xH2O MW461.30(anhydrous basis) 25g
4-Methoxybenzenediazonium tetrafluoroborate CAS NO.459-64-3 CH3OC6H4N2BF4 MW221.95 25g
3,5-Dichlorophenyldiazonium tetrafluoroborate CAS NO.350-67-4 C6H3Cl2N2.BF4 MW260.81 25g
4-Nitrobenzenediazonium tetrafluoroborate; Azoic Diazo No.37; Fast Red GG salt CAS NO.456-27-9 C6H4N3O2·BF4 MW236.92 25g
2-Amino-6-chlorobenzoic acid CAS NO.2148-56-3 H2NC6H3(Cl)CO2H MW171.58, Assay 99%(HPLC), form Solid powder,, color light yellow to beige 25g
5-Amino-2-nitrobenzoic acid,2-Nitro-5-aminobenzoic acid, 3-Carboxy-4-nitroaniline, NSC 74455, H2NC6H3(NO2)CO2H CAS NO.13280-60-9 MW182.13 Beilstein:2107512 EC No.236-283-4 UNSPSC Code:12352106, Assay 99%, form Powder or Crystals solid, color Light Yellow to Yellow to Beige, reaction suitability reaction type: solution phase peptide synthesis, mp236-238 °C (lit.), application(s) peptide synthesis, Application Reactant for:Two-component dendritic chain reactions, Enzymic activation of hydrophobic self-immolative dendrimers, Preparation of insulin receptor tyrosine kinase activator, Preparation of polymer-bound diazonium salts using Merrifield resin-bound piperazine 25g
3,5-Dichlorobenzoic acid CAS NO.51-36-5 Cl2C6H3CO2H MW191.01 25g
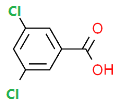
3,3',5,5'-Tetramethylbenzidine CAS NO.54827-17-7 [-C6H2(CH3)2-4-NH2]2 MW240.34 25g
3,3',5,5'-Tetramethylbenzidine dihydrochloride CAS NO.64285-73-0 C16H20N2.2HCl MW313.27 25g
5-Bromo-4-chloro-3-indolyl phosphate p-toluidine salt CAS NO.6578-6-9 C8H6BrClNO4P.C7H9N MW433.62 25g
5-Bromo-4-chloro-3-indolyl phosphate disodium salt CAS NO.102185-33-1 C8H4BrClNO4P.2Na MW370.43 25g
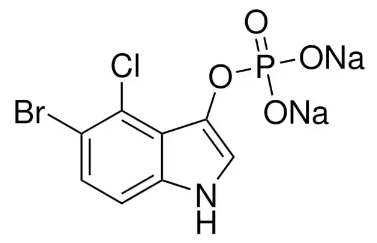
S-Acetylthiocholine iodide CAS NO.1866-15-5 C7H16INOS MW289.18 25g
2,6-Dichloroindophenol sodium salt, 2,6-Dichlorophenolindophenol sodium salt hydrate, Sodium 2,6-dichloroindophenolate hydrate, 2,6-Dichloro-N-(4-hydroxyphenyl)-1,4-benzoquinoneimine sodium salt, 2,6-Dichlorophenolindophenol sodium salt hydrate, DCIP, CAS NO.620-45-1 1266615-56-8 MW290.08 (anhydrous basis) C12H6Cl2NNaO2·xH2O Beilstein:3641229 EC No.210-640-4 UNSPSC Code:12171500, Assay 97%, form powder, color Very Light Blue to Very Dark Blue to dark green, solubility H2O: soluble 10 mg/mL, clear, blue to very deep blue, suitability suitable for vitamin C determination, application(s) diagnostic assay manufacturing, food and beverages, hematology, histology, storage temp.room temp, General description 2,6-Dichloroindophenol sodium salt hydrate is a blue redox dye. Application 2,6-Dichloroindophenol sodium salt hydrate is suitable for vitamin C determination. 2,6-Dichloroindophenol (DCIP) may be used in the following studies: As redox dye to investigate the efficiency of gold nanoparticles (Au-NP) for enzymatic activity of glucose oxidase (GOx). To compose UVB specific dosimeter. Quantification of vitamin C (L-ascorbic acid) in fruit juices. As substrate in NQO1 assay to investigate the liver cytosolic NQO1 activity spectrophotometrically. Mitochondrial succinate CoQ oxidoreductase (complex II) activity assay. 25g
3-[(3-Cholamidopropyl)dimethylammonio]propanesulfonate CAS NO.75621-03-3 25g
dimethylammonio]propanesulfonate.png)
Tetrazolium blue chloride CAS NO.1871-22-3 C40H32Cl2N8O2 MW727.64 25g
Nitrotetrazolium blue chloride CAS NO.298-83-9 C40H30N10O6·2Cl MW817.64 25g
Iodonitrotetrazolium chloride CAS NO.146-68-9 C19H13ClIN5O2 25g
Tetrazolium violet CAS NO.1719-71-7 C23H17ClN4 MW384.86 25g
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt CAS NO.150849-52-8 25g

2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt CAS NO. 193149-74-5 25g

2-phenyl-3-(1-naphthyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt 25g

2-(4-nitrophenyl)-3-(1-naphthyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt 25g

2-(4-nitrophenyl)-3-(4,5-Dimethyl-2-thiazolyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt 25g

2-phenyl-3-(4,5-Dimethyl-2-thiazolyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt 25g

3,3'-Dianisole-4,4'-bis((3-(2,4-disulfophenyl)-5-phenyl)tetrazolium sodium salt) 25g

2-(benzothiazole-2-yl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)tetrazolium monosodium salt wavelength 600-750nm 25g

2-(benzothiazole-2-yl)-3-(4-iodophenyl)-5-(2,4-disulfophenyl)tetrazolium monosodium salt wavelength 600-750nm 25g

2-(benzothiazole-2-yl)-3-(2-methoxy-4-nitrophenyl)-5-(2,4-disulfophenyl)tetrazolium monosodium salt wavelength 600-750nm 25g

3-Methylphenylhydrazine hydrochloride; m-Tolylhydrazine hydrochloride CAS NO.637-04-7 CH3C6H4NHNH2·HCl MW158.63 25g

2,3-Dimethylphenylhydrazine hydrochloride CAS NO.56737-75-8 25g
4-Methoxyphenylhydrazine hydrochloride CAS NO.19501-58-7 C7H11ClN2O MW174.63 25g
3-Bromophenylhydrazine hydrochloride CAS NO.27246-81-7 BrC6H4NHNH2·HCl MW223.50 25g
3-Chlorophenylhydrazine hydrochloride CAS NO.2312-23-4 ClC6H4NHNH2·HCl MW179.05 25g
2-Chlorophenylhydrazine hydrochloride CAS NO.41052-75-9 ClC6H4NHNH2·HCl MW179.05 25g

3-Fluorophenylhydrazine hydrochloride CAS NO.2924-16-5 FC6H4NHNH2·HCl MW162.59 25g
4-Fluorophenylhydrazine hydrochloride CAS NO.823-85-8 FC6H4NHNH2·HCl MW162.59 25g
3',3'',5',5''-Tetrachlorophenol-3,4,5,6-tetrabromosulfophthalein 25g
3,4,5,6-Tetrabromophenolsulfonephthalein CAS NO.77172-72-6 25g
Indocyanine green CAS NO.3599-32-4 1g
2-Hydrazinopyridine dihydrochloride CAS NO.62437-99-4 25g
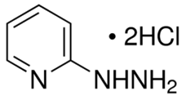
omega-Aminoalkanesulfonic acids
N-Phenylglycine potassium salt
1-Butanesulfonic acid sodium salt CAS NO.2386-54-1
1-Propanesulfonic acid sodium salt CAS NO.14533-63-2
2,4-Dinitrophenylpyridinum chloride
2-Naphthylhydrazine
1,1,2-trimethylbenz[e]indole CAS NO.41532-84-7
Glutaconic aldehyde dianilide monohydrochloride, N-[5-(Phenylamino)-2,4-pentadienylidene]aniline monohydrochloride, Glutaconaldehydedianil Hydrochloride,Glutaconic aldehyde dianilide hydrochloride, C6H5NHCH=CHCH=CHCH=NC6H5·HCl CAS NO.1497-49-0 MW284.78 EC No.216-094-3 UNSPSC Code:12171500, Assay 98%(HPLC), form powder or chunks, color Red to Very Dark Red, mp173-175 °C (lit.), application(s) diagnostic assay manufacturing hematology histology, storage temp.room temp, Application Tricarbocyanine laser dye intermediate

3,4,5,6-Tetrabromo-2-sulfobenzoic acid anhydride
p-Cresylphenyl acetate CAS NO.101-94-0
3-Chloro-2-hydroxy-1-propane sulfonic acid, sodium salt CAS NO.143218-48-8
2-Methylindole-3-carboxaldehyde CAS NO.5416-80-8
Phenylhydrazine hydrochloride series
3,5-Dichlorobenzoyl chloride CAS NO.2905-62-6 C7H3Cl3O MW209.46
2,6-Dinitrobenzaldehyde CAS NO.606-31-5
3,3'-Diaminobenzidine tetrahydrochloride
Phenyl 3,4-diaminobenzoate
3,3'-Diaminobenzidine CAS NO.91-95-2 (Cu Element: Not-Detected)
3-Maleimidopropionic acid CAS NO.7423-55-4
3-Maleimidopropionic acid N-hydroxysuccinimide ester CAS NO.55750-62-4
4-(4-Maleimidophenyl)butyric acid N-hydroxysuccinimidyl ester CAS NO.79886-55-8
4-(N-Maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester CAS NO.64987-85-5
3-Maleimidobenzoic acid N-hydroxysuccinimide ester CAS NO. 58626-38-3
11-(Maleimido)undecanoic acid N-succinimidyl ester CAS Number: 87981-04-2
Maleimidoacetic acid N-hydroxysuccinimide ester CAS NO.55750-61-3
6-Maleimidohexanoic acid N-hydroxysuccinimide ester CAS NO.55750-63-5
Bisindolylmaleimide IV CAS NO. 119139-23-0
Bisindolylmaleimide V CAS NO. 113963-68-1
BES
ACES
CHES
MES
PIPES disodium salt
HEPES sodium salt
CAPS
MOPS, MPS
HEPPS
POPSO
3-[N-Bis(hydroxyethyl)amino]-2-hydroxypropyl sulfonic acid
3-[N-Tris(hydroxymethyl)methylamino]-2-hydroxypropane sulfonic acid
N-(Hydroxyethyl)piperazine-N'-2-hydroxypropyl sulfonic acid
4-[N-Bis(hydroxyethyl)amino]-2-hydroxypropyl sulfonic acid
3-(N-Morpholino)-2-hydroxypropyl sulfonic acid
D-Galactose CAS NO.59-23-4
1-Naphthyl acetate
2-Naphthyl acetate
1-Naphthylphosphate sodium salt
2-Naphthylphosphate sodium salt
p-Nitrophenyl acetate
4-Nitrophenylphosphate disodium salt
Phenylphosphate disodium salt
Amino acid derivatives
Acid fuchsin CAS NO.3244-88-0
Alizarin CAS NO.72-48-0
Alizarin complexone CAS NO.3952-78-1
Alizarin red S CAS NO.130-22-3
Alizarin yellow GG CAS NO.584-42-9
Alizarin yellow R CAS NO.2243-76-7
Basic fuchsin CAS NO.569-61-9
Bromochlorophenol blue CAS NO.2553-71-1
Bromochlorophenol blue sodium salt CAS NO.102185-52-4
Bromocresol green CAS NO.76-60-8
Bromocresol green sodium salt CAS NO.62625-32-5
Bromocresol purple CAS NO.115-40-2
Bromocresol purple sodium salt CAS NO.62625-28-9
Bromophenol blue sodium salt CAS 62625-28-9
Bromophenol red sodium salt CAS NO.102185-50-2
Bromopyrogallol red CAS NO.16574-43-9
Bromothymol blue CAS NO.76-59-5
Bromothymol blue sodium salt CAS NO.34722-90-2
Bromoxylenol blue CAS NO.40070-59-5
Calcein CAS NO.1461-15-0
o-Chlorophenol red CAS NO.4430-20-0
o-Cresolphthalein complexone CAS NO.2411-89-4
o-Cresolphthalein complexone disodium salt CAS NO.94442-10-1
m-Cresol purple CAS NO.2303-01-7
m-Cresol purple sodium salt CAS NO.62625-29-0
Cresol red CAS NO.1733-12-6
Cresol red sodium salt CAS NO.62525-29-0
o-Cresolphthalein monophosphate disodium salt
o-Cresolphthalein monophosphate dipotassium salt
o-Cresolphthalein monophosphate diammonium salt
o-Cresolphthalein monophosphate dilithium salt
o-Cresolphthalein monophosphate magnesium salt
o-Cresolphthalein monophosphate calcium salt
o-Cresolphthalein monophosphate di(cyclohexylammonium) salt
o-Cresolphthalein monophosphate N-methylglucammonium salt
o-Cresolphthalein monophosphate tetramethylethylenediammonium salt
Crystal violet CAS NO.548-62-9
Eosin Y free acid CAS NO.15086-94-9
Eosin Y disodium salt CAS NO.17372-87-1
Evans blue CAS NO.314-13-6
Fluorescein CAS NO.2321-07-5
Fluorescein sodium salt CAS NO.518-47-8
Gentian violet CAS NO.548-62-9
Glycine cresol red sodium salt CAS NO.77031-64-2
Methyl blue CAS NO.28983-56-4
Methyl green CAS NO.7114-03-6
Methyl orange CAS NO.547-58-0
Methyl red CAS NO.493-52-7
Methyl violet CAS NO.8004-87-3
Methylene blue CAS NO.7220-79-3
Methylene green zinc chloride double salt CAS NO.224967-52-6
Napthol green B CAS NO.19381-50-1
1-Naphtholphthalein monophosphate
1-Naphtholphthalein monophosphate disodium salt
Oil red O CAS NO.1320-06-5
Orange II CAS NO.633-96-5
Orange IV CAS NO.554-73-4
Orange G CAS NO.1936-15-8
1,10-Phenanthroline CAS NO.5144-89-8
1,10-Phenanthroline hydrochloride monohydrate CAS NO.3829-86-5
Phenolphthalein-3,3'-bis(methylimino)diacetic acid CAS NO.77031-64-2
Phenolphthalein monophosphate bis(ethanolammonium) salt
Phenolphthalein diacetate
Phenolphthalein diphosphate CAS NO.2090-82-6
Phenolphthalein diphosphate calcium salt CAS NO.94465-66-4
Phenolphthalein bisphosphate pyridine salt CAS NO.267240-23-3
Monoethanolamine phenolphthalein diphosphate
Phenol red CAS NO.143-74-8
Phenol red sodium salt CAS NO.34487-61-1
Pyrocatechol violet CAS NO.115-41-3
Tetrabromophenol blue CAS NO.4430-25-5
Tetrabromophenol blue sodium salt CAS NO.108321-10-4
3',3'',5',5''-Tetrachlorophenol-3,4,5,6-tetrabromosulfophthalein
3,4,5,6-Tetrabromophenolsulfonephthalein CAS NO.77172-72-6
3',3'',5',5''-Tetraiodophenolsulfophthalein CAS 4430-24-4
Thymolphthalein CAS NO.125-20-2
Thymolphthalein complexone CAS NO.1913-93-5
Thymolphthalein complexone sodium salt CAS NO.85409-48-9
Thymolphthalein monophosphoric acid diammonium salt CAS NO.51027-02-2
Thymolphthalein monophosphate 2-amino-2-methyl-1,3-propanediol salt CAS NO.52279-66-0
1-Naphtholphthalein monophosphate disodium salt pure
1-Naphtholphthalein monophosphate magnesium salt pure
(Sulfonated)(naphthol)phenol(sulfo)phthalein, or monophosphate, or complexone, or their salts
NBT CAS NO.298-83-9
Tetranitrobluetetrazolium chloride CAS NO.1184-43-6
XTT CAS NO.111072-31-2
other Tetrazolium salts
IR-820 CAS NO.172616-80-7
3-(N-Tosyl-L-alaninyloxy)-5-phenylpyrrole CAS NO.221446-55-5
2-Methoxy-4-morpholinobenzenediazonium chloride zinc chloride double salt CAS NO.67801-08-5
(2E)-2-[(2E,4E)-5-[1-[6-[3-[4-amino-1-[(2R,4R,5R)-4-hydroxy-5-[[hydroxy-[hydroxy(phosphonooxy)phosphoryl]oxyphosphoryl]oxymethyl]oxolan-2-yl]-2-oxopyrimidin-5-yl]prop-2-ynylamino]-6-oxohexyl]-3,3-dimethyl-5-sulfoindol-1-ium-2-yl]penta-2,4-dienylidene]-1-ethyl-3,3-dimethylindole-5-sulfonate N,N-diethylethanamine; CY5 5-(3-Aminopropargyl)-2'-deoxycytidine 5'-triphosphate triethylammonium salt 95%min(HPLC) C45H57N6O20P3S2.xC6H15N MW1159.029306 (free acid basis)

(2E)-2-[(2E,4E)-5-[1-[6-[3-[4-amino-1-[(2R,4R,5R)-4-hydroxy-5-[[hydroxy-[hydroxy(phosphonooxy)phosphoryl]oxyphosphoryl]oxymethyl]oxolan-2-yl]-2-oxopyrimidin-5-yl]prop-2-ynylamino]-6-oxohexyl]-3-methyl-3-(4-sulphobutyl)-5-sulfoindol-1-ium-2-yl]penta-2,4-dienylidene]-1,3-bis(4-sulphobutyl)-3-methylindole-5-sulfonate;N,N-diethylethanamine 95%min(HPLC) C53H73N6O29P3S5.x(C2H5)3N MW1511.436946 (free acid basis)
(2E)-2-[(2E,4E)-5-[1-[6-[3-[4-amino-1-[(2R,4R,5R)-4-hydroxy-5-[[hydroxy-[hydroxy(phosphonooxy)phosphoryl]oxyphosphoryl]oxymethyl]oxolan-2-yl]-2-oxopyrimidin-5-yl]prop-2-ynylamino]-6-oxohexyl]-3,3-dimethylindol-1-ium-2-yl]penta-2,4-dienylidene]-1-ethyl-3,3-dimethylindole;N,N-diethylethanamine 95%min(HPLC) C45H58N6O14P3.xC6H15N MW999.908846 (free acid basis)

C5-propargylamino-dCTP 5-(3-aminopropynyl)-2'-deoxycytidine-5'-triphosphate 5-(3-aminoprop-1-ynyl)-2'-deoxycytidine-5'-triphosphate Aminopropargyl dCTP C12H15O13N4P3Li4 MW543.95546
C5-propargylamino-dUTP 5-(3-aminopropynyl)-2’-deoxyuridine-5’-triphosphate 5-(3-aminoprop-1-ynyl)-2’-deoxyuridine-5’-triphosphate Aminopropargyl dUTP C12H14O14N3P3Li4 MW544.940266

Cy3 Carboxylic acid;Cyanine3 carboxylic acid;Cy3-carboxylic acid chloride;6-[3,3-dimethyl-2-[3-(1,3,3-trimethylindol-1-ium-2-yl)prop-2-enylidene]indol-1-yl]hexanoic acid chloride "2089112-98-9 1032678-01-5 (chloride) 1251915-29-3" C30H37ClN2O2 493.08876
Cy3 carboxylic acid (Propane Sulfo);3-[2-[(E,3E)-3-[1-(5-carboxypentyl)-3,3-dimethylindol-2-ylidene]prop-1-enyl]-3,3-dimethylindol-1-ium-1-yl]propane-1-sulfonate C32H40N2O5S 564.74608
.png)
Cy3 acid (mono SO3);Cy3 acid(butane sulfo);4-[(2E)-2-[(E)-3-[1-(5-carboxypentyl)-3,3-dimethylindol-1-ium-2-yl]prop-2-enylidene]-3,3-dimethylindol-1-yl]butane-1-sulfonate 644979-14-6 C33H42N2O5S 578.77296
.png)
Sulfo-Cy3-carboxylic acid;Cy3 carboxylic acid, trisulfo; 1-(5-carboxypentyl)-2-[(E,3E)-3-[3,3-dimethyl-5-sulfo-1-(3-sulfopropyl)indol-2-ylidene]prop-1-enyl]-3,3-dimethylindol-1-ium-5-sulfonate 1252582-04-9 C32H40N2O11S3 724.87448
diSulfo-Cy3 carboxylic acid; sodium;(2E)-1-(5-carboxypentyl)-3,3-dimethyl-2-[(E)-3-(1,3,3-trimethyl-5-sulfonatoindol-1-ium-2-yl)prop-2-enylidene]indole-5-sulfonate C30H35N2NaO8S2 638.738348
Cy3-SE; (2Z)-2-[(E)-3-[1-[6-(2,5-dioxopyrrolidin-1-yl)oxy-6-oxohexyl]-3,3-dimethyl-5-sulfoindol-1-ium-2-yl]prop-2-enylidene]-1-ethyl-3,3-dimethylindole-5-sulfonate 146368-16-3 C35H41N3O10S2 727.85676
Sulfo-Cyanine3-alkyne;Cy3-YNE;Sulfo-Cy3-YNE;2-[3-[3,3-dimethyl-1-[6-oxo-6-(prop-2-ynylamino)hexyl]-5-sulfoindol-1-ium-2-yl]prop-2-enylidene]-1-ethyl-3,3-dimethylindole-5-sulfonate 1010386-62-5 C34H41N3O7S2 667.84756
Cy3 azide;N-(3-azidopropyl)-6-[3,3-dimethyl-2-[3-(1,3,3-trimethylindol-1-ium-2-yl)prop-2-enylidene]indol-1-yl]hexanamide chloride 2183440-48-2 C33H43ClN6O 575.19696
Cy3 alkyne;6-[(2E)-3,3-dimethyl-2-[(E)-3-(1,3,3-trimethylindol-1-ium-2-yl)prop-2-enylidene]indol-1-yl]-N-prop-2-ynylhexanamide chloride C33H40ClN3O 530.15292
Sulfo-Cy3.5-carboxylic acid tripotassium salt;tripotassium;(2E)-3-(5-carboxypentyl)-1,1-dimethyl-2-[(E)-3-(1,1,3-trimethyl-6,8-disulfonatobenzo[e]indol-3-ium-2-yl)prop-2-enylidene]benzo[e]indole-6,8-disulfonate C38H37K3N2O14S4 991.275760
Cy3.5 Carboxylic acids;6-[1,1-dimethyl-2-[(E)-3-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)prop-2-enylidene]benzo[e]indol-3-yl]hexanoic acid chloride 1144107-79-8 C38H41ClN2O2 593.20852
Cy3.5 NHS ester;(2,5-dioxopyrrolidin-1-yl) 6-[(2E)-1,1-dimethyl-2-[(E)-3-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)prop-2-enylidene]benzo[e]indol-3-yl]hexanoate chloride C42H44ClN3O4 690.28188
Cy5-YNE; (2Z)-2-[(2E,4E)-5-[3,3-dimethyl-1-[6-oxo-6-(prop-2-ynylamino)hexyl]-5-sulfoindol-1-ium-2-yl]penta-2,4-dienylidene]-1-ethyl-3,3-dimethylindole-5-sulfonate 1345823-20-2 C36H43N3O7S2 693.88544
Cy5-SE;Sulfo-Cy5-SE;Cy5-NHS ester;2-[5-[1-[6-(2,5-dioxopyrrolidin-1-yl)oxy-6-oxohexyl]-3,3-dimethyl-5-sulfoindol-1-ium-2-yl]penta-2,4-dienylidene]-1-ethyl-3,3-dimethylindole-5-sulfonate 146368-14-1 C37H43N3O10S2 753.89464
Cy5 alkyne;Cyanine5 alkyne;6-[3,3-dimethyl-2-[5-(1,3,3-trimethylindol-1-ium-2-yl)penta-2,4-dienylidene]indol-1-yl]-N-prop-2-ynylhexanamide chloride 1223357-57-0 C35H42ClN3O 556.1908
Cy5 carboxylic acid;6-[(2E)-3,3-dimethyl-2-[(2E,4E)-5-(1,3,3-trimethylindol-1-ium-2-yl)penta-2,4-dienylidene]indol-1-yl]hexanoic acid; C32H39N2O(2)+ 483.67394
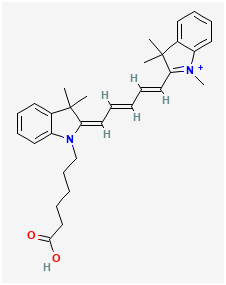
sulfo-Cy5.5-carboxylic acid tripotassium salt;tripotassium;(2E)-3-(5-carboxypentyl)-1,1-dimethyl-2-[(2E,4E)-5-(1,1,3-trimethyl-6,8-disulfonatobenzo[e]indol-3-ium-2-yl)penta-2,4-dienylidene]benzo[e]indole-6,8-disulfonate 2183440-69-7 C40H39K3N2O14S4 1017.31364
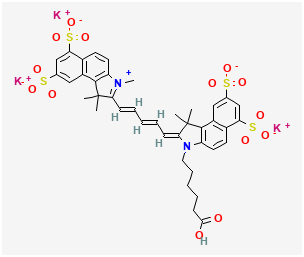
Cy5.5 DBCO;3-[6-[[3-(2-azatricyclo[10.4.0.04,9]hexadeca-1(16),4,6,8,12,14-hexaen-10-yn-2-yl)-3-oxopropyl]amino]-6-oxohexyl]-2-[(1E,3E,5E)-5-(3-ethyl-1,1-dimethyl-6,8-disulfobenzo[e]indol-2-ylidene)penta-1,3-dienyl]-1,1-dimethyl-8-sulfobenzo[e]indol-3-ium-6-sulfonate 1857352-95-4 C59H58N4O14S4 1175.39208
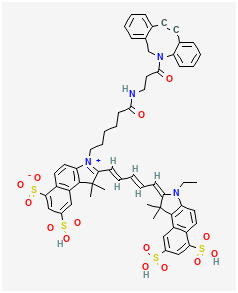
Cy55 Azide;N-(3-azidopropyl)-6-[(2E)-1,1-dimethyl-2-[(2E,4E)-5-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)penta-2,4-dienylidene]benzo[e]indol-3-yl]hexanamide chloride C43H49ClN6O 701.3546

CY5.5 azide (quadruple SO3);CY5.5 azide (tetra SO3);3-[6-(3-azidopropylamino)-6-oxohexyl]-2-[(1E,3E,5Z)-5-(3-ethyl-1,1-dimethyl-6,8-disulfobenzo[e]indol-2-ylidene)penta-1,3-dienyl]-1,1-dimethyl-8-sulfobenzo[e]indol-3-ium-6-sulfonate 1955527-41-9 C44H50N6O13S4 999.177640
.png)
Cy7 NHS ester chloride; Cy7 NHS ester; (2,5-dioxopyrrolidin-1-yl)-6-[(2E)-3,3-dimethyl-2-[(2E)-2-[3-[(E)-2-(1,3,3-trimethylindol-1-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]indol-1-yl]hexanoate chloride C41H48ClN3O4 682.30264
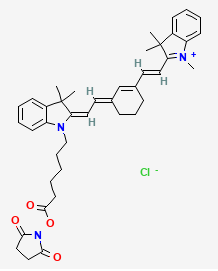
Cy7-SE(double sulfo);(2Z)-2-[(2E,4E,6E)-7-[1-[6-(2,5-dioxopyrrolidin-1-yl)oxy-6-oxohexyl]-3,3-dimethyl-5-sulfoindol-1-ium-2-yl]hepta-2,4,6-trienylidene]-1-ethyl-3,3-dimethylindole-5-sulfonate;Cy7-NHS ester(double sulfo) 477908-53-5 C39H45N3O10S2 779.93252
.png)
Cy7-NHS ester tetrafluoroborate;(2,5-dioxopyrrolidin-1-yl) 6-[(2E)-3,3-dimethyl-2-[(2E)-2-[3-[(E)-2-(1,3,3-trimethylindol-1-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]indol-1-yl]hexanoate tetrafluoroborate 2408482-09-5 C41H48BF4N3O4 733.654553
Sulfo-Cy7 NHS ester (2*SO3); (2E)-1-[6-(2,5-dioxopyrrolidin-1-yl)oxy-6-oxohexyl]-3,3-dimethyl-2-[(2E)-2-[3-[(E)-2-(1,3,3-trimethyl-5-sulfonatoindol-1-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]indole-5-sulfonate C41H46N3O10S2- 804.96246
.png)
Sulfo-Cy7-NHS ester (trisulfo);2-[(E)-2-[(3E)-3-[(2E)-2-[3,3-dimethyl-5-sulfo-1-(3-sulfopropyl)indol-2-ylidene]ethylidene]cyclohexen-1-yl]ethenyl]-1-[6-(2,5-dioxopyrrolidin-1-yl)oxy-6-oxohexyl]-3,3-dimethylindol-1-ium-5-sulfonate C43H51N3O13S3 914.08836
.png)
CY7.5 AZIDE;N-(3-Azidopropyl)-6-[1,1-dimethyl-2-[7-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)hepta-2,4,6-trienylidene]benzo[e]indol-3-yl]hexanamide chloride 2183440-52-8 C45H51ClN6O 727.39248
Cy7.5 Acid(mono SO3);ICG-carboxylic acid;4-[2-[7-[3-(5-carboxypentyl)-1,1-dimethylbenzo[e]indol-3-ium-2-yl]hepta-2,4,6-trienylidene]-1,1-dimethylbenzo[e]indol-3-yl]butane-1-sulfonate C45H50N2O5S 730.96848
.png)
Sulfo-Cy7 amine;Sulfo-Cyanine7 amine;1-[6-(6-azaniumylhexylamino)-6-oxohexyl]-3,3-dimethyl-2-[2-[3-[2-(1,3,3-trimethyl-5-sulfonatoindol-1-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]indole-5-sulfonate; 2236573-39-8 C43H58N4O7S2 807.088280
Cy7 amine;Cy7-Amine chloride hydrochloride;6-[6-[(2E)-3,3-dimethyl-2-[(2E)-2-[3-[(E)-2-(1,3,3-trimethylindol-1-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]indol-1-yl]hexanoylamino]hexylazanium dichloride 1650559-73-1 C43H60Cl2N4O 719.88116
Cy7.5-Amine chloride hydrochloride;6-[6-[(2E)-1,1-dimethyl-2-[(2E)-2-[3-[(E)-2-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]benzo[e]indol-3-yl]hexanoylamino]hexylazanium dichloride 2104005-17-4 C51H64Cl2N4O 820.00092
Sulfo-Cyanine5 amine;Sulfo-Cy5 amine;1-[6-(6-azaniumylhexylamino)-6-oxohexyl]-3,3-dimethyl-2-[5-(1,3,3-trimethyl-5-sulfonatoindol-1-ium-2-yl)penta-2,4-dienylidene]indole-5-sulfonate 2183440-44-8 C38H52N4O7S2 740.98564
N-(Azide-PEG3)-N'-(Amine-C3-Amide-PEG4)-Cy5;N-(azide-PEG3)-N'-(Amine-C3-PEG4)-Cy5;N-(3-aminopropyl)-3-[2-[2-[2-[2-[2-[(1E,3E,5E)-5-[1-[2-[2-[2-(2-azidoethoxy)ethoxy]ethoxy]ethyl]-3,3-dimethylindol-2-ylidene]penta-1,3-dienyl]-3,3-dimethylindol-1-ium-1-yl]ethoxy]ethoxy]ethoxy]ethoxy]propanamide chloride C47H70ClN7O8 896.56788
-N'-(Amine-C3-Amide-PEG4)-Cy5.png)
CY5-Amine chloride hydrochloride;6-[6-[(2E)-3,3-dimethyl-2-[(2E,4E)-5-(1,3,3-trimethylindol-1-ium-2-yl)penta-2,4-dienylidene]indol-1-yl]hexanoylamino]hexylazanium dichloride 1807529-70-9 C38H54Cl2N4O 653.77852

Bis-(N,N'-amine-PEG3)-Cy5;2-[2-[2-[2-[(2E)-2-[(2E,4E)-5-[1-[2-[2-[2-(2-aminoethoxy)ethoxy]ethoxy]ethyl]-3,3-dimethylindol-1-ium-2-yl]penta-2,4-dienylidene]-3,3-dimethylindol-1-yl]ethoxy]ethoxy]ethoxy]ethanamine chloride C41H61ClN4O6 741.4114
-Cy5.png)
Cy5-PEG5-amine HCl;2-[2-[2-[2-[2-[2-[6-[3,3-dimethyl-2-[5-(1,3,3-trimethylindol-1-ium-2-yl)penta-2,4-dienylidene]indol-1-yl]hexanoylamino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethylazanium dichloride C44H66Cl2N4O6 817.9368
Cy5 N-succinimidyl ester DIPEA Salt (1:1);CY5-SE/DIPEA;1-[6-(2,5-dioxopyrrolidin-1-yl)oxy-6-oxohexyl]-2-[(1E,3E,5Z)-5-(1-ethyl-3,3-dimethyl-5-sulfoindol-2-ylidene)penta-1,3-dienyl]-3,3-dimethylindol-1-ium-5-sulfonate;N-ethyl-N-propan-2-ylpropan-2-amine 1033006-65-3 C45H62N4O10S2 883.14024
Cy5 Amine;6-[6-[(2E)-3,3-dimethyl-2-[(2E,4E)-5-(1,3,3-trimethylindol-1-ium-2-yl)penta-2,4-dienylidene]indol-1-yl]hexanoylamino]hexylazanium C38H54N4O+2 582.87312
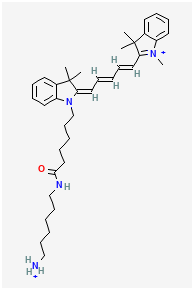
Cy3 amine;Cy3-Amine chloride hydrochloride;6-[6-[(2E)-3,3-dimethyl-2-[(E)-3-(1,3,3-trimethylindol-1-ium-2-yl)prop-2-enylidene]indol-1-yl]hexanoylamino]hexylazanium dichloride 2247688-56-6 C36H52Cl2N4O 627.74064
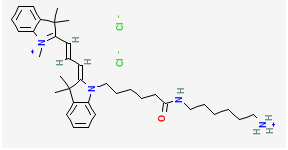
Sulfo-Cy3 amine;(2E)-1-[6-(6-azaniumylhexylamino)-6-oxohexyl]-3,3-dimethyl-2-[(E)-3-(1,3,3-trimethyl-5-sulfonatoindol-1-ium-2-yl)prop-2-enylidene]indole-5-sulfonate C36H50N4O7S2 714.94776
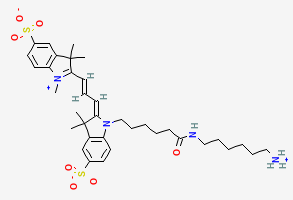
Cy5.5 carboxylic acid;6-[1,1-dimethyl-2-[(2E,4E)-5-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)penta-2,4-dienylidene]benzo[e]indol-3-yl]hexanoate 1144107-80-1 C40H42N2O2 582.78576
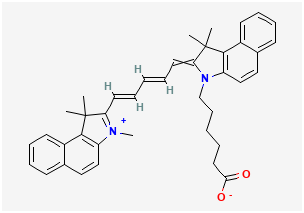
Cy5.5 NHS ester;(2,5-dioxopyrrolidin-1-yl) 6-[(2E)-1,1-dimethyl-2-[(2E,4E)-5-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)penta-2,4-dienylidene]benzo[e]indol-3-yl]hexanoate chloride 2054318-82-8 C44H46ClN3O4 716.31976
Based on BODIPY fluorescent probe HGLGE2 for carbonic anhydrases CA I, CA II and CA XIII detection
Based on BODIPY fluorescent probe HGLGE3 for carbonic anhydrases CA I, CA II and CA XIII detection

Near-infrared Ratio-Type Heptamethine Cyanine fluorescent probe QDKJCY-OP with mitochondrial specificity for highly sensitive and selective detection of alkaline phosphatase (ALP)
Near-infrared Ratio-Type Heptamethine Cyanine fluorescent probe QDKJCY-TYR for detection of tyrosinase (TYR)
Near-infrared Ratio-Type Heptamethine Cyanine fluorescent probe QDKJCY-GGT for detection of gamma-glutamyltransferase (GGT)

Two-photon chloro-coumarin-hemicyanine fluorescent probe CQTPP-CBSCSE for detection of H2S and Cys
1,8-Naphthalimide-based fluorescent probe YNNUH1 for detection of uridine diphosphate (UDP) and imaging UDP in living cells
7-Hydroxycoumarin-based fluorescent probe HDLGH3 for detection of histidine
1,8-Naphthalimide-based fluorescent probe YN1 for detection of GMP, TMP and UMP
Benzopyran nitrile-based fluorescent probe YNA1 for detection of nitroreductase(NTR)
Naphthalimide-based fluorescent probe HDLGProbe12 for detection of gamma-glutamyltransferase(GGT) and imaging GGT in living cells
Near-infrared tyrosinase responsive fluorescent probe HNLGNBR-AP for detection of tyrosinase (TYR) and used in Melanoma Imaging
Near-infrared tyrosinase responsive fluorescent probe HEBSYProbe4 for detection of nitroreductase (NTR)
Near-infrared two-photon fluorescent probe QFSFDCM-CES2 for detection of carboxylesterase 2(CES2)
Camptothecin-based fluorescent probe YNARP1 for detection of azo reductase and targeting hypoxia tumors
Fluorescent probe HDLGProbe2 for detection of phenylalanine ammonia lyase(PAL)
ESIPT based ratio-type fluorescent probe QDKJHBTP-mito for detection of alkaline phosphatase(ALP)
ESIPT based ratio-type fluorescent probe QDKJHBTP+Cu2+ for detection of acetylcholinesterase(AChE)
Fluorescent probe QDKJP1 for detection of butyrylcholinesterase(BChE)
ICT based Fluorescent probe HNMsr-P for bioimaging of methionine sulfoxide reductase
BODIPY-based "Turn-On" and ratiometric Fluorescent probe HBBODIPY-C-Leu for detection of leucine aminopeptidase(LAP) in cancer cells
Fluorescein PET-based "Turn-On" Fluorescent probe HBFluorescein-Ph for detection of tyrosinase(TYR) in living cells and imaging
Coumarin-based "Turn-On" and ratiometric Fluorescent probe HBCoumarin-Gal for detection of beta-galactosidase(beta-Gal)
NIR ICT-based "Turn-On" Fluorescent probe HNSFDXMP for detection of alkaline phosphatase(ALP) in vivo
NIR visible "Turn-On" Fluorescent probe HNSFDXM-bgal for detection of beta-galactosidase(beta-gal) in living cells
NIR large stokes shift "Turn-On" Fluorescent probe HNSFDCM-CHO-bgal for detection of beta-galactosidase(beta-gal) in living cells
Fluorescent probe XBCA for detection of lysosomal pH and beta-galactosidase(beta-gal) in cancer cells

Two-photon Fluorescent probe HNQ3CA-P for detection and imaging of quinone oxidoreductase in living cells and tissues
Two-photon Fluorescent probe HNCDAN for detection and imaging of histone deacetylases in living cells and tissues
Near infrared Fluorescent probe HNNIR-PAP for detection and imaging of Prolyl aminopeptidase(PAP) in living cells
Near infrared "turn-on" Fluorescent probe HNNap-F for detection and hypoxia imaging of intracellular Furin

Novel Near infrared Fluorescent probe HNHD-F for real-time imaging and dynamic monitoring of Furin in tumor tissues and in vivo
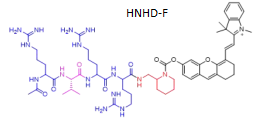
Novel Near infrared "tunr-on" Fluorescent probe HNMito-Bcy-GGT for detection of mitochondrial gamma-Glutamyl transpeptidase(GGT) in living cells
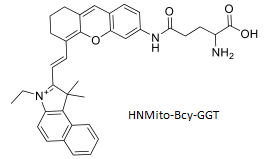
Novel Near infrared Fluorescent probe HNAMVF for detection of mitochondrial vicinal dithiol-containing proteins(VDPs) in living cells
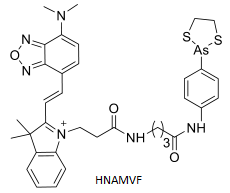
Novel Near infrared "off-on" Fluorescent probe HNDCI-Ac for detection of cysteine(Cys) in biological systems
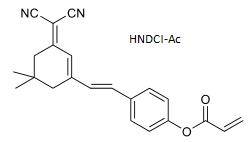
Highly selective colorimetric dual-signal Fluorescent probe HNSFProbe3 for detection of cysteine(Cys) in serum

Carbazole-based dual-site two-photon Fluorescent probe JNCA-TPP for detection of mitochondrial targeting RSH in living cells and tissues
Phenanthraquinone and imibazole hybrid "off-on" Fluorescent probe JNPI for detection and imaging of Cu2+ and cysteine in living cells and tissues
Dual-site two-photon Fluorescent probe JNTP-PMVC for detection and imaging of lysosome and lysosome's H2S in cells and tissues
Bodipy-based thiol Fluorescent probe ZNP8 for detection and imaging of biothiols(Cys, Hcy, GSH,etc) in cells and tissues
1,8-Naphthalimide-based Fluorescent probe NJSFABPA for detection and imaging of cysteine(Cys) in cells and tissues
Benzothiazole-based biothiols Fluorescent probe NJSFBPBC for detection and bioimaging of cysteine(Cys) in living cells, with low cytotoxicity
Isophorone-based Fluorescent probe NJSFDP-NIR for detection and bioimaging of cysteine(Cys) in living cells
3-Hydroxyflavone-based Fluorescent probe NJSFSCA with large Stokes-shift for detection and bioimaging of cysteine(Cys) in living cells
Coumarin-based two recognition fluorephore ratiometric Fluorescent probe SDACCA for detection and bioimaging of cysteine(Cys), Cys/Hcy,GSH, in calf serum and living cells
Coumarin-acrylate-based enhanced Fluorescent probe SDACA for detection and bioimaging of cysteine(Cys) in calf serum and living cells

Coumarin-acrylate-based ratiometric Fluorescent probe SDDPCA for detection and bioimaging of cysteine(Cys) in calf serum and living cells
Coumarin-acrylate-based enhanced Fluorescent probe SDPPN for detection and bioimaging of GSH in calf serum and living cells
Coumarin-dinitrosulfonyl-based enhanced Fluorescent probe SDDPCS for detection and bioimaging of biothiols in calf serum and living cells
Coumarin-quinone-based enhanced Fluorescent probe SDPBQC for detection and bioimaging of GSH in calf serum and living cells

Borate-1,8-Naphthalimide-based enhanced Fluorescent probe HNSFBN for detection of acetylcholinesterase(AChE)
3-Hydroxybenzylamine as recognition fluorephore "turn-on" Fluorescent probe HZSFW-NBD for rapid detection of Tyrosinase(TYR) without interfering in reactive oxygen species
BODIPY-based ratiometric Fluorescent probe HZSFB-BODIPY for rapid detection of Tyrosinase(TYR)
Fluorescent probe WHCompound1 for detection of Thrombin
Two-photon Fluorescent probe WHCompound6 for rapid detection of Tyrosinase(TYR)
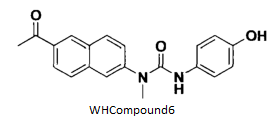
Ratiometric and mitochondria NIR Fluorescent probe QFSFMitoCy-NH2 for levels of MAO-B and synergistic imaging of MAO-B and its contribution to oxidative stress in cells and in mice models
Ratiometric and mitochondria NIR Fluorescent probe QFSFMitoHCy-NH2 for levels of MAO-B and synergistic imaging of MAO-B and its contribution to oxidative stress in cells and in mice models
Ratiometric and mitochondria NIR Fluorescent probe QFSFMitoCy-NMe2 for synergistic detection of MAOs and its contribution to oxidative stress in cells and in mice models
Ratiometric and mitochondria NIR Fluorescent probe QFSFMitoHCy-NMe2 for levels of MAOs and synergistic imaging of MAOs and it contribution to oxidative stress in cells and in mice models
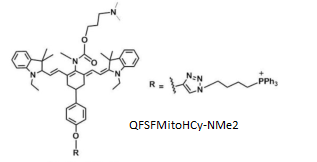
Effective and Sensitive Fluorescent probe 4-Methyl-7-(3-aminopropoxy)coumarin for the activities of MAO-A and MAO-B
coumarin.png)
High sensitivity and high selectivity Fluorescent probe HNNIR-hNQO1 for the activities of NAD(P)H:quinone oxidoreductase isozyme 1(HNQO1) in vivo and in vitro
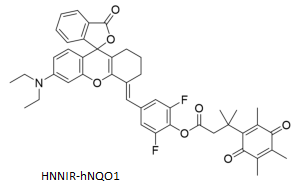
Rhodamine and L-alanine based Fluorescent probe HNRhodamine-APN for detection and imaging of aminopeptidase N(APN) in vivo
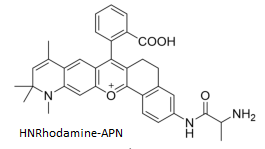
NBD-Cl and glutathione based Fluorescent probe HNNBD-GSH for detection of gamma-Glutamyl transpeptidase(GGT) in living cells
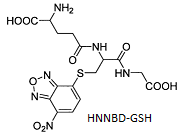
Coumarin-based Fluorescent probe HNNQO1P1 for detection of NAD(P)H:quinone oxidoreductase 1(NQO1) in living cells
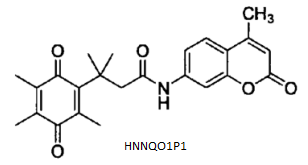
GCTPOC-based two photon Fluorescent probe JNG-GAL for detection and imaging of beta-gal in cells and tissues
ESIPT-based Fluorescent probe JNESIPT-GAL for detection of beta-gal in living cells
Red light emitting Two-photon Fluorescent probe JNTR-G for detection and bioimaging of beta-gal in vitro and in vivo

ESIPT Ratiometric Fluorescent probe 2-(benzo[d]thiazol-2-yl)phenyldihydrogen phosphate (HNSFPBT) for detection of endogenous alkaline phosphatase(ALP) in living cells and fluorescence imaging of ALP in LO2 and human hepatocellular carcinoma(HepG2)
Near-infrared Fluorescent probe HNSFDCM-beta-gal for monitoring of endogenous activity of beta-galactosidase(beta-gal) in living cells and the fluorescence imaging of ovarian cancer cells (SKOV-3)

IR780-based High selectivity and sensitivity Fluorescent probe NJLGIRPSM+Ir3+ for detection of GSH and imaging in HepG2
High selectivity and sensitivity Fluorescent probe NJLGTRDM-F for detection of Cys and imaging in HepG2
ICT based benzoxadiazole Fluorescent probe HNSFNOB for detection of cysteine(Cys)
Benzoxazole-based two-photon Fluorescent probe DLLGBABO for detection and imaging of carboxylesterase 1(CE1) in living cells and tissue slices
Highly selective long-wavelength Fluorescent probe DLLGTCFB for detection of carboxylesterase 2(CE2) in complex biological systems
ESIPT-based Ratiometric Fluorescent probe DLLG3-AF for detection of carboxylesterase 2(CE2) in complex biological systems
ICT-based highly sensitive NIR Fluorescent probe DLLGDDAP for detection of human serum albumin(HSA) in plasma and hepatocytes
ICT-based resorufin-derived high selectivity and high sensitivity Fluorescent probe DLLGCHPO for evaluation of cytochrome CYP1A1 in human liver microsomal
Coumarin-based high selectivity and high sensitivity Fluorescent probe BJGYF2 for detection of tyrosinase
Phenothiazine-based NIR Fluorescent probe BJGYMB1 for detection of tyrosinase and killing melanoma cells under 655nm laser irradiation
ESIPT-based two photon NIR Fluorescent probe HNTP-NTR-1 for detection and imaging of nitroreductase(NTR)
ESIPT-based two photon NIR Fluorescent probe HNTP-CE-1 for detection and imaging of carboxylesterase 1(CE-1)
BODIPY-based high selectivity and high sensitivity Fluorescent probe DLLGProbe1 for detection and imaging of carboxylesterase 1(CE-1) in living cells and tissues.
Dicyanoisophorone-derived and camptothecin as anticancer drug Fluorescent probe anti-tumor theranostic ProdrugT1-O-Drug for detection and imaging of cysteine(Cys).
Dual-site Fluorescent probe FDOCl-N-NBD for detection and imaging of endogenous homocysteine(Hcy) and HOCl in cancer cells.
Dual-site Fluorescent probe SDSFCNN for detection and imaging of nitroreductase (NRT) and human quinone reductase 1 (hNQO1) in cancer cells.
Hypoxia reactive diagnostic prodrug Fluorescent probe HBFDU-BCM-NO2 coupled by active drug floxuridine(FDU) for detection and imaging of cancer cells.
Bisfunctional Fluorescent probe JSProbe1 for detection and imaging of thiols and Primary aliphatic amines(PAA) in human blood serum
L-Tryptophan-derived NIR Fluorescent probe SDSFTrp-Cy for detection and bioimaging of ozone(O3) in cancers and mitochondria
Hemicyanine and NBD-derived NIR Fluorescent probe HB-NBD for detection of biothiols and imaging of mitochondrial biothiols in HepG2 cells
Hemicyanine and 2,4-dinitrophenyl ether-derived NIR Fluorescent probe HS for detection of thiophenol in HepG2 cells
WZB-117-conjugated fluorophore NIR Fluorescent probe TJHXH-05 for GLUT1 inbibitor in vitro and in vivo cancer theranostics and image-based quantitative high resolution tumor-targeted cancer diagnosis
Cy.7.Cl and TPP-conjugated fluorophore NIR Fluorescent probe ZKYCyssTPP for detection of GSH and imaging and high-efficient PDT therapeutica in vivo with tumor-targeted robust phototherapy
Acenaphthol[1,2-b]quinonxaline-based fluorescence-enhancing Fluorescent probe HDLGA5 for detection of hypoxic cells in solid tumors
benzo-imidazole isoquinoline ketone fluorescence-quenched Fluorescent probe HDLGB2 for detection of hypoxic cells in solid tumors
Naphthalimide moiety fluorescence-enhancing bioreductive Fluorescent probe HDLGA4 for detection of hypoxic cells in solid tumors, CHO cells, 95D cells, V79 cells

Benzo-imidazole iso-quinoline ketone and benzo[1,2,5]oxadiazole bioreductive Fluorescent probe HDLGD2 for detection of hypoxic cells in solid tumors, V79 cells
water soluble Benzo-imidazole iso-quinoline ketone fluorescence-incresed bioreductive Fluorescent probe HDLGF3 for detection of hypoxic cells in solid tumors, V79 cells
Zinc 4,8,12,16-Tetra(2',2',2'-trifluoroethoxy)phthalocyanine Fluorescent probe HDLG2a for detection of cells in solid tumors with DNA photocleaving ability and photodynamic tumor therapy with emulsifying agents
Aggregation-induced emission NIR Fluorescent probe SZQMTP for detection and imaging of endogenous alkaline phosphate(ALP) in tumors in vitro and in vivo
NIR Fluorescent probe SZIndol-Glu for detection and imaging of endogenous (gamma-Glutamyltranspeptidase)GGT in tumors in vitro and in vivo, which facilitates the image-guided surgical resection
2-(1H-benzo[d]imidazol-2-yl)phenylboronic acid Fluorescent probe for detection of D-fructose
Pyrazoline-based highly sensative and selective Fluorescent probe SDProbe4 for detection of glutathione(GSH)
Novel carbazole two-photon ratiometric Fluorescent probe SDCAEI for detection and bioimage of mitochondria in cells
Stilbene Hydrophobic two-photon Fluorescent probe DLLGDCN2 for detection and bioimage of human serum albumin (HSA) in living tissues
Reaction-based 2,4-dinitrophenyl and coumarin derivative Fluorescent probe DLLGXT for detection and bioimage of H2S in cells
Reaction-based 4-chloro-7-nitrobenzene-2-oxo-1,3-diazole(NBDCl) derivative Fluorescent probe DLLGZH for detection and bioimage of formaldehyde(FA) in cells
Se-based Ratiometric two-photon highly selective and highly sensitive enhancing Fluorescent probe SDSFCoum-Se for detection and bioimage of HClO in cells
PET and ESIPT-based 2,4-dinitrophenyl ether endoplasmic reticulum two-photon Fluorescent probe SDSFER-H2S for detection and bioimage of H2S in cells
Cy7.Cl-based near-infrared Fluorescent probe ZZCy-NphS for detection and bioimage of hypochlorous acid(HClO) in living cells, and MPO enzyme assay
Fluorescein-based Selenium reversible Fluorescent probe ZZProbe1 for detection and bioimage of hypochlorous acid(HClO) in living cells, for oxidation-reduction process between HClO and GSH

2,7-Dichlorofluorescein-based Selenium reversible Fluorescent probe ZZProbe2 for detection and bioimage of hypochlorous acid(HClO) in living cells, for the oxidation-reduction process between HClO and GSH
4-Methylamino-1,8-naphthalimide as fluorophore turn-on two-photon rapid highly-selective highly-sensitive Fluorescent probe HBMZHCA-Green for detection and bioimage of hypochlorous acid(HClO) in living cells
Seminaphthorhodafluor as fluorophore turn-on two-photon rapid highly-selective highly-sensitive ratiometric Fluorescent probe HBMZYG-Red for detection and bioimage of hypochlorous acid(HClO) in living substances in vivo
Rhodamine-like mitochondrial-targeted two-photon Fluorescent probe HBMZES-HOCl for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) and localizing mitochondrial cells
Aza-coumarin based fast response high sensitivity turn-on PET-based Fluorescent probe DLLGAC-ClO for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) in cells
3-Pyrrole BODIPY enhancing-PET-basedoff-on rapid high selectivity ratiometric Fluorescent probe DLLGBRClO for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) in cells
BODIPY-based mitochondria-locatable off-on rapid high selectivity Fluorescent probe DLLGMitoClO1 for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) in mitochrondria in living cells and organisms
BODIPY-based mitochondria-locatable off-on rapid high selectivity Fluorescent probe DLLGMitoClO2 for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) in cells and organisms
BODIPY-based rapid high selectivity ratiometric significant blueshift Fluorescent probe DLLGBODIPYWCHO for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) in mitochrondria in living cells and organisms
Selenamorpholine sensatized rapid high selectivity Fluorescent probe DLLGSeCY7 for detection and bioimage of exogenous and endogenous hypochlorous acid(HClO) in mitochrondria in living cells and organisms
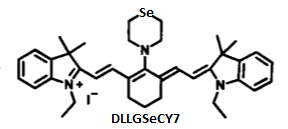
Phenyl-acetyl-pyrazoline-based Fluorescent probe SDPhenyl-Zn for detection and bioimage of Zn(II) in living cells and organisms
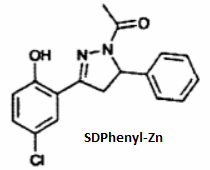
Naphthalene-acetyl-pyrazoline-based Fluorescent probe SDNaphthalene-Zn for detection and bioimage of Zn(II) in living cells and organisms
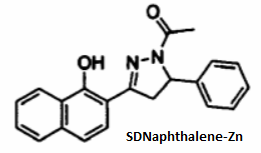
Rhodamine-derivative water-soluble Fluorescent probe AHSFProbe1 for detection and imaging of PH in intra/extra living cells and organisms
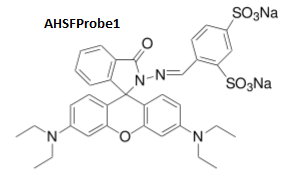
Benzothiazole conjugated with pyridine by vinyl ICT-based large Stokes shift Fluorescent probe NJBTP2 for detection and imaging of PH in intra/extra living cells
Rhodamine-derivative with spirolactam ICT-based lysosome-targeted Fluorescent probe HNLyso-Rh-H2S for detection and imaging of H2S in lysosomes under acid enviroment
Novel fluorophore by coumarin and merocyanine through an ethylene group ICT-based ratiometric and colorimetric NIR Fluorescent probe HNCou-Hem-H2S for detection and imaging of H2S in biosystem
Novel courmarin fluorophore Fluorescent probe STProbe1 for detection and imaging of NH2NH2 in living cells
Novel diethylin courmarin fluorophore on-off response acidic PH Fluorescent probe STProbe4 for detection and imaging of pH in lisosome pKa=4.61
Novel fluorescein dilaurate Fluorescent probe TJfluorescein dilaurate for detection of lipoidase at 0.1mg/ml with an accuracy of 98.5%
Novel Rhodamine B ester fluorophore Fluorescent probe TJMethyl rhodamine B ester for detection of DNA by resonance light scattering with detection limit 5.8ng/ml
Rhodamine conjugated naphthalene chromophore sensitively and selectively ratiometric FRET fluorescent probe DLLGRNP1 for detection and imaging of Fe3+ in mitochondria in living cells with detection limit 6.93*10-6M
Benzocyanine fluorophore conjugated with 4-nitrobenzyl group ICT mechanism mitochondrial Targeting highly selective and highly sensitive Fluorescent probe HNBICP for detection and imaging of Nitroreductase(NTR) I and II with detection limit 1.9ng/mL
4-chloro-7-diethylamino coumarin fluorophore conjugated with benzoindole mitochondria targeted ICT mechanism ratiometric high selectivity and high sensitivity fluorescent probe HNP1 for detection and imaging of mitochondrial HSO3-/SO3(2-) exogenous and endogenous in cells mitochondria with detection limit 0.16μmol/L
Rhodamine B fluorophore acylhydrazine conjugated with 4-Bromo-2-hydroxyphenyl group high selectivity and high sensitivity fluorescent probe DHRHBr for detection and imaging of Cu2+ Al3+ Ca2+ with detection limit 1.7*10-7M 2.8*10-7M 1.0*10-8M respectively, while complex DHRHBr-Al3+ can detect endogenous L-phenylalanine(LPA) in cells with detection limit 3.0*10-8M
Rhodamine B fluorophore acylhydrazine conjugated with N,N-dimethylpyridylamine group high selectivity and high sensitivity fluorescent probe DHRACD for detection and imaging of Cu2+ in living cells with detection limit 1.1*10-9M
Rhodamine B fluorophore acylhydrazine conjugated with cyanuric group high selectivity and high sensitivity large Stokes shift fluorescent probe DHFHCS for detection and imaging of Al3+ Zn2+ in living cells with detection limit 5.37*10-8M 7.9*10-8M
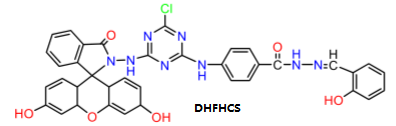
Piperazine-substituted tricarbocyanine high selectivity and high sensitivity NIR fluorescent probe XMProbe21 for detection and imaging of biological thiols at PH8.4

1,8-Naphthalimide-derivative acrylhydrazide high selectivity and high sensitivity NIR ratiometric fluorescent probe XMProbe1 for detection and imaging of HClO in human glioma cells
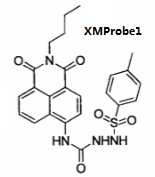
Hemicyanine conjugated NBD group high selectivity and high sensitivity NIR fluorescent probe JSNIR-NBD for detection and imaging of biothiols(H2S Cys Hcy GSH) in living cells
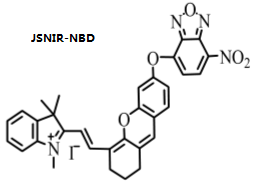
Hemicyanine conjugated 2,4-dinitrophenyl group high selectivity and high sensitivity NIR fluorescent probe JSArSH-probe for detection and imaging of thiophenols in living cells
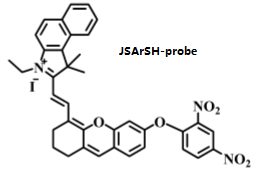
Benzothiazole-derived cyanine high selectivity and high sensitivity NIR fluorescent probe HBDLCy-SN for detection and imaging of endogenous and exogenous peroxynitrite(ONOO-) in living cells with detection limit 26nM
Tricarbocyanine-based piperazine-methyl-pyridine high selectivity and high sensitivity NIR fluorescent probe XTProbe1 for detection and imaging of trivalent chromium ions(Cr3+) in living cells with detection limit 2.5*10-8M
Benzoindole-ethylene 3,4-dihydroxyphenyl group high selectivity and high sensitivity NIR fluorescent probe TJLGL2 for detection and imaging of Hydrogen Sulfate Ion (HSO4-) in living cells with detection limit 8.39*10-7M
Cy7 derived resorufin high selectivity and high sensitivity colormetric and rationmetric NIR fluorescent probe XMProbe-biothiols for detection and imaging of biothiols(Cys/Hcy GSH)
Ethylene glycol oligomer-modified NIR unsymmetrical squaraine high selectivity and high sensitivity fluorescent probe HNLGUSSQ-Leu for detection and imaging of endogenous leucine aminopeptidase(LAP)

Silicon phthalocyanine complexs axially modified by hydrophilic groups very low toxicity DLLGPc2 for photodynamic therapy(PDT)
Novel Lipid solubility and water solubility 3,5-Bis-oxy-methylene-carborane benzoic acid showes the ability to deliver 10B atoms to HePG2 cells with high selectivity and less cytotoxic ideal new boron delivery agent for BNCT therapy cancer cells
Novel carborane based on COX-2 inhibitors N,N-bis-methylene-carborane celecoxib showed high boron loading, high COX-2 selective inhibition and low cytoxicity for BNCT therapy in cancer cells
Novel quinolizium hemicyanine dye high selectivity and high sensitivity fluorescent probe TJLGProbe L for detection of DNA and imaging of nucleic acids with cells with detect limit 8.04*10-8M monitoring the deoxyribonuclease activity and hydrolysis process
Novel cyanine dye high selectivity and high sensitivity fluorescent probe TJLGTPTP for detection of DNA and selective imaging of nucleic acids within dead cells with detect limit 1.13*10-7M monitoring the deoxyribonuclease activity and hydrolysis process DNA staining in electrophoresis
Novel water soluble nitrobenzyl moieties cyanine high selectivity and high sensitivity off-on NIR fluorescent probe QHNTR-BI for detection and imaging of nitroreductase(NTR) in cells
Novel dihydroxynaphthalene ethylene bridged 4-methylpyridine cyanine dye NIR intracelluar low cytoxicity mitochondria targeted nucleic acid fluorescent probe TJLGCy-P for detection of DNA with detection limit 6.9ng/L strong electrostatic interaction with ct-DNA and RNA
Novel heptamethine indocyanine bridged 2,4-dinitrosulfonyl NIR intracelluar very low cytoxicity fluorescent probe LZCyDNS for detection and imaging of biothiols (GSH Cys and Hcy) in cells in vivio and tissues
Novel hemicyanine bridged acryl NIR intracelluar very low cytoxicity off-on high selectivity high sensitivity fast response fluorescent probe LZCyA for detection and imaging of biothiols (Cys) in cells with detection limit 0.16μM
Novel hemicyanine bridged acetyl NIR intracelluar low cytoxicity sole selectivity high sensitivity efficiency ratiometric colormetric turn-on ICT mechanism fluorescent probe LZCyJ for detection and imaging of N2H4 in cells and tissues in vivo with detection limit 0.17μM
Novel hemicyanine bridged Diphenylphosphinyl NIR intracelluar low cytoxicity high selectivity high sensitivity rapid response colormetric fluorescent probe LZCyR for detection and imaging of superoxide radical anion(O2.-) in tissues in vivo with detection limit 9.9nM

Novel hemicyanine coumarin and quinoline as fluorophore p-hydroxybenzyl as linker t-butyldiphenylchlorosilane as reaction site NIR intracelluar low cytoxicity high selectivity high sensitivity rapid response fluorescent probe JNMito-PF for detection and imaging of fluoro anion(F-) in biosystems in vivo with detection limit 0.7712μmol and for detection of polarity in cells
Novel Rhodamine B-based schiff base intracelluar low cytoxicity high selectivity high sensitivity off-on fluorescent probe NJSFProbe1 for detection and imaging of Cu2+ and L-Methionine in biosystems